| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:30:07 UTC |
|---|
| Update Date | 2016-11-09 01:17:28 UTC |
|---|
| Accession Number | CHEM022367 |
|---|
| Identification |
|---|
| Common Name | Cabazitaxel |
|---|
| Class | Small Molecule |
|---|
| Description | A tetracyclic diterpenoid that is 10-deacetylbaccatin III having O-methyl groups attached at positions 7 and 10 as well as an O-(2R,3S)-3--2-hydroxy-3-phenylpropanoyl group attached at position 13. Acts as a microtubule inhibitor, binds tubulin and promotes microtubule assembly and simultaneously inhibits disassembly. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
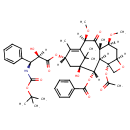
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Cabazitaxelum | ChEBI | | Jevtana | Kegg | | Taxoid XRP6258 | HMDB | | Kabazitaxel | MeSH |
|
|---|
| Chemical Formula | C45H57NO14 |
|---|
| Average Molecular Mass | 835.932 g/mol |
|---|
| Monoisotopic Mass | 835.378 g/mol |
|---|
| CAS Registry Number | 183133-96-2 |
|---|
| IUPAC Name | (1S,2S,3R,4S,7R,9S,10S,12R,15S)-4-(acetyloxy)-15-{[(2R,3S)-3-{[(tert-butoxy)carbonyl]amino}-2-hydroxy-3-phenylpropanoyl]oxy}-1-hydroxy-9,12-dimethoxy-10,14,17,17-tetramethyl-11-oxo-6-oxatetracyclo[11.3.1.0³,¹⁰.0⁴,⁷]heptadec-13-en-2-yl benzoate |
|---|
| Traditional Name | cabazitaxel |
|---|
| SMILES | [H][C@@](O)([C@@H](NC(=O)OC(C)(C)C)C1=CC=CC=C1)C(=O)O[C@@]1([H])C[C@@]2(O)[C@@]([H])(OC(=O)C3=CC=CC=C3)[C@]3([H])[C@@]4(CO[C@]4([H])C[C@]([H])(OC)[C@@]3(C)C(=O)[C@]([H])(OC)C(=C1C)C2(C)C)OC(C)=O |
|---|
| InChI Identifier | InChI=1S/C45H57NO14/c1-24-28(57-39(51)33(48)32(26-17-13-11-14-18-26)46-40(52)60-41(3,4)5)22-45(53)37(58-38(50)27-19-15-12-16-20-27)35-43(8,36(49)34(55-10)31(24)42(45,6)7)29(54-9)21-30-44(35,23-56-30)59-25(2)47/h11-20,28-30,32-35,37,48,53H,21-23H2,1-10H3,(H,46,52)/t28-,29-,30+,32-,33+,34+,35-,37-,43+,44-,45+/m0/s1 |
|---|
| InChI Key | BMQGVNUXMIRLCK-OAGWZNDDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzazepines. These are organic compounds containing a benzene ring fused to an azepine ring (unsaturated seven-membered heterocycle with one nitrogen atom replacing a carbon atom). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzazepines |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Benzazepines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzazepine
- Azepine
- Imidazole-4-carbonyl group
- Aryl-aldehyde
- N-substituted imidazole
- Piperidine
- Benzenoid
- Imidazole
- Azole
- Heteroaromatic compound
- Tertiary aliphatic amine
- Tertiary amine
- Azacycle
- Organic oxide
- Amine
- Organooxygen compound
- Organonitrogen compound
- Aldehyde
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0560-4210021920-cfe7bca2e816c750b927 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a6r-8720051900-f9830c3fa7f065c90a7e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-6900480000-305aa5a26586210ddaee | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-6520060940-56520d63332c6b16b932 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05fr-7200090210-2482a20c2ef7e8b60901 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0ab9-9300050000-51a40ac30e5efd33b739 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-057j-0390350010-f59c18861840c9edd5ac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-06w9-2790000100-ef16e22c38ab8ef57b0b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0aor-3910001000-ed8392d91c8613a519fd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-071i-6770090850-e3ffa0bcc67aa375fef1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0btl-7750090510-a6339783dd488a76cf29 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00b9-9820000000-5b21c3d5026480e5af06 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB06772 |
|---|
| HMDB ID | HMDB0015672 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Cabazitaxel |
|---|
| Chemspider ID | 8029779 |
|---|
| ChEBI ID | 63584 |
|---|
| PubChem Compound ID | 9854073 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. https://www.ncbi.nlm.nih.gov/pubmed/?term=21174534 | | 2. https://www.ncbi.nlm.nih.gov/pubmed/?term=21339064 | | 3. https://www.ncbi.nlm.nih.gov/pubmed/?term=21406025 | | 4. https://www.ncbi.nlm.nih.gov/pubmed/?term=21448449 | | 5. https://www.ncbi.nlm.nih.gov/pubmed/?term=21455038 | | 6. https://www.ncbi.nlm.nih.gov/pubmed/?term=21461278 | | 7. https://www.ncbi.nlm.nih.gov/pubmed/?term=21463139 | | 8. https://www.ncbi.nlm.nih.gov/pubmed/?term=21695098 | | 9. https://www.ncbi.nlm.nih.gov/pubmed/?term=21734586 | | 10. https://www.ncbi.nlm.nih.gov/pubmed/?term=21748753 | | 11. https://www.ncbi.nlm.nih.gov/pubmed/?term=21770474 | | 12. https://www.ncbi.nlm.nih.gov/pubmed/?term=22048000 | | 13. https://www.ncbi.nlm.nih.gov/pubmed/?term=22079047 | | 14. https://www.ncbi.nlm.nih.gov/pubmed/?term=22111007 | | 15. https://www.ncbi.nlm.nih.gov/pubmed/?term=22229405 | | 16. Galsky MD, Dritselis A, Kirkpatrick P, Oh WK: Cabazitaxel. Nat Rev Drug Discov. 2010 Sep;9(9):677-8. doi: 10.1038/nrd3254. | | 17. Nightingale G, Ryu J: Cabazitaxel (jevtana): a novel agent for metastatic castration-resistant prostate cancer. P T. 2012 Aug;37(8):440-8. | | 18. Kort A, Hillebrand MJ, Cirkel GA, Voest EE, Schinkel AH, Rosing H, Schellens JH, Beijnen JH: Quantification of cabazitaxel, its metabolite docetaxel and the determination of the demethylated metabolites RPR112698 and RPR123142 as docetaxel equivalents in human plasma by liquid chromatography-tandem mass spectrometry. J Chromatogr B Analyt Technol Biomed Life Sci. 2013 Apr 15;925:117-23. doi: 10.1016/j.jchromb.2013.02.034. Epub 2013 Mar 5. | | 19. Simons K, Toomre D: Lipid rafts and signal transduction. Nat Rev Mol Cell Biol. 2000 Oct;1(1):31-9. | | 20. Watson AD: Thematic review series: systems biology approaches to metabolic and cardiovascular disorders. Lipidomics: a global approach to lipid analysis in biological systems. J Lipid Res. 2006 Oct;47(10):2101-11. Epub 2006 Aug 10. | | 21. Sethi JK, Vidal-Puig AJ: Thematic review series: adipocyte biology. Adipose tissue function and plasticity orchestrate nutritional adaptation. J Lipid Res. 2007 Jun;48(6):1253-62. Epub 2007 Mar 20. | | 22. Lingwood D, Simons K: Lipid rafts as a membrane-organizing principle. Science. 2010 Jan 1;327(5961):46-50. doi: 10.1126/science.1174621. | | 23. The lipid handbook with CD-ROM |
|
|---|