| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:28:12 UTC |
|---|
| Update Date | 2016-11-09 01:17:28 UTC |
|---|
| Accession Number | CHEM022322 |
|---|
| Identification |
|---|
| Common Name | Ticarcillin |
|---|
| Class | Small Molecule |
|---|
| Description | A penicillin compound having a 6beta-[(2R)-2-carboxy-2-thiophen-3-ylacetyl]amino side-group. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
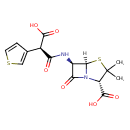
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (2S,5R,6R)-6-{[(2R)-2-carboxy-2-thiophen-3-ylacetyl]amino}-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid | ChEBI | | alpha-Carboxy-3-thienylmethylpenicillin | ChEBI | | Ticarcilina | ChEBI | | Ticarcilline | ChEBI | | Ticarcillinum | ChEBI | | Ticillin | Kegg | | (2S,5R,6R)-6-{[(2R)-2-carboxy-2-thiophen-3-ylacetyl]amino}-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylate | Generator | | a-Carboxy-3-thienylmethylpenicillin | Generator | | Α-carboxy-3-thienylmethylpenicillin | Generator | | TIPC | HMDB | | Ticarcillin supplement | HMDB | | Ticar | HMDB | | Ticarcillin disodium | HMDB | | Ticarpen | HMDB | | SmithKline beecham brand OF ticarcillin disodium salt | HMDB | | Tarcil | HMDB | | GlaxoSmithKline brand OF ticarcillin disodium | HMDB | | CSL Brand OF ticarcillin disodium | HMDB | | Disodium, ticarcillin | HMDB | | GlaxoSmithKline brand OF ticarcillin disodium salt | HMDB | | SmithKline beecham brand OF ticarcillin disodium | HMDB |
|
|---|
| Chemical Formula | C15H16N2O6S2 |
|---|
| Average Molecular Mass | 384.427 g/mol |
|---|
| Monoisotopic Mass | 384.045 g/mol |
|---|
| CAS Registry Number | 34787-01-4 |
|---|
| IUPAC Name | (2S,5R,6R)-6-[(2R)-2-carboxy-2-(thiophen-3-yl)acetamido]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carboxylic acid |
|---|
| Traditional Name | ticarcillin |
|---|
| SMILES | [H][C@]12SC(C)(C)[C@@H](N1C(=O)[C@H]2NC(=O)[C@H](C(O)=O)C1=CSC=C1)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C15H16N2O6S2/c1-15(2)9(14(22)23)17-11(19)8(12(17)25-15)16-10(18)7(13(20)21)6-3-4-24-5-6/h3-5,7-9,12H,1-2H3,(H,16,18)(H,20,21)(H,22,23)/t7-,8-,9+,12-/m1/s1 |
|---|
| InChI Key | OHKOGUYZJXTSFX-KZFFXBSXSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dipeptides. These are organic compounds containing a sequence of exactly two alpha-amino acids joined by a peptide bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Dipeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-dipeptide
- Penicillin
- N-acyl-alpha amino acid or derivatives
- Alpha-amino acid or derivatives
- Penam
- Dicarboxylic acid or derivatives
- 1,3-dicarbonyl compound
- Beta-lactam
- Tertiary carboxylic acid amide
- Thiazolidine
- Thiophene
- Heteroaromatic compound
- Azetidine
- Secondary carboxylic acid amide
- Carboxamide group
- Lactam
- Hemithioaminal
- Thioether
- Azacycle
- Organoheterocyclic compound
- Dialkylthioether
- Carboxylic acid
- Hydrocarbon derivative
- Organopnictogen compound
- Organic oxygen compound
- Organonitrogen compound
- Organooxygen compound
- Organic nitrogen compound
- Carbonyl group
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9546000000-6f3ebd66c2d3c7a7045c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-022c-9122010000-a4161ee57059b48e816b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03fr-1977000000-70bc3924298ffb1f2db8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0400-1931000000-8c1407e95f1db40189a2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0cfu-5910000000-19e60a838e854593fc5c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0191000000-4523daaf1e52e8059ce2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0007-0392000000-6946a7c83efe338aae0d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00el-8961000000-fb2a198cbb7c67eecf26 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0029000000-0985d9aea35e6323bc40 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00ku-0935000000-0bd2fefb341089ef6fab | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-1900000000-49f18e0899a4acafa1fc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00m0-0809000000-7ddcf6f962ff0e233398 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-4901000000-ae5815191ae219d3bea5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9300000000-acfad4e268b76916d13e | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01607 |
|---|
| HMDB ID | HMDB0015545 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Ticarcillin |
|---|
| Chemspider ID | 33876 |
|---|
| ChEBI ID | 9587 |
|---|
| PubChem Compound ID | 36921 |
|---|
| Kegg Compound ID | C07139 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|