| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:28:09 UTC |
|---|
| Update Date | 2016-11-09 01:17:28 UTC |
|---|
| Accession Number | CHEM022320 |
|---|
| Identification |
|---|
| Common Name | Pivmecillinam |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
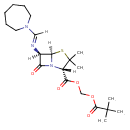
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Pivmecillinam | Kegg | | PMPC | Kegg | | Coactabs | Kegg | | [(2S,5R,6R)-6-[(e)-[(Azepan-1-yl)methylidene]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carbonyloxy]methyl 2,2-dimethylpropanoic acid | Generator |
|
|---|
| Chemical Formula | C21H33N3O5S |
|---|
| Average Molecular Mass | 439.570 g/mol |
|---|
| Monoisotopic Mass | 439.214 g/mol |
|---|
| CAS Registry Number | 32886-97-8 |
|---|
| IUPAC Name | [(2S,5R,6R)-6-[(E)-[(azepan-1-yl)methylidene]amino]-3,3-dimethyl-7-oxo-4-thia-1-azabicyclo[3.2.0]heptane-2-carbonyloxy]methyl 2,2-dimethylpropanoate |
|---|
| Traditional Name | pivmecillinam |
|---|
| SMILES | [H]\C(=N/[C@]1([H])C(=O)N2[C@]1([H])SC(C)(C)[C@]2([H])C(=O)OCOC(=O)C(C)(C)C)N1CCCCCC1 |
|---|
| InChI Identifier | InChI=1S/C21H33N3O5S/c1-20(2,3)19(27)29-13-28-18(26)15-21(4,5)30-17-14(16(25)24(15)17)22-12-23-10-8-6-7-9-11-23/h12,14-15,17H,6-11,13H2,1-5H3/b22-12+/t14-,15+,17-/m1/s1 |
|---|
| InChI Key | NPGNOVNWUSPMDP-UTEPHESZSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as alpha amino acid esters. These are ester derivatives of alpha amino acids. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Alpha amino acid esters |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-amino acid ester
- Penam
- Azepane
- Acylal
- Dicarboxylic acid or derivatives
- Beta-lactam
- Tertiary carboxylic acid amide
- Thiazolidine
- Azetidine
- Carboxamide group
- Carboxylic acid ester
- Lactam
- Acetal
- Amidine
- Formamidine
- Carboxylic acid amidine
- Azacycle
- Organoheterocyclic compound
- Dialkylthioether
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Carboximidamide
- Hemithioaminal
- Thioether
- Organic oxygen compound
- Organonitrogen compound
- Organooxygen compound
- Organopnictogen compound
- Carbonyl group
- Organic oxide
- Hydrocarbon derivative
- Organic nitrogen compound
- Aliphatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aliphatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0lkc-0933100000-207d607d624521759c4a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-066r-2911000000-e698803b355747dc85ad | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9400000000-4c251e4b9870f1bb8e2e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0911000000-0118b6d73875ca106ecc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-1931000000-ced5e83f734188289e15 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00c0-9612000000-5d2b3043f24082963710 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|