| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:27:04 UTC |
|---|
| Update Date | 2016-11-09 01:17:27 UTC |
|---|
| Accession Number | CHEM022292 |
|---|
| Identification |
|---|
| Common Name | Tasosartan |
|---|
| Class | Small Molecule |
|---|
| Description | Tasosartan is a long-acting angiotensin II (AngII) receptor blocker. Its long duration of action has been attributed to its active metabolite enoltasosartan. It is used to treat patients with essential hypertension. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
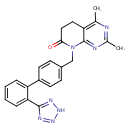
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| ANA-756tasosartan | HMDB | | 5,8-Dihydro-2,4-dimethyl-8-((2'-(1H-tetrazol-5-yl)(1,1'-biphenyl)-4-yl)methyl)pyrido(2,3-D)pyrimidin-7(6H)-one | HMDB | | ANA-756 | HMDB | | Taso-sartan | HMDB | | ANA 756 | HMDB |
|
|---|
| Chemical Formula | C23H21N7O |
|---|
| Average Molecular Mass | 411.459 g/mol |
|---|
| Monoisotopic Mass | 411.181 g/mol |
|---|
| CAS Registry Number | 145733-36-4 |
|---|
| IUPAC Name | 2,4-dimethyl-8-({4-[2-(2H-1,2,3,4-tetrazol-5-yl)phenyl]phenyl}methyl)-5H,6H,7H,8H-pyrido[2,3-d]pyrimidin-7-one |
|---|
| Traditional Name | tasosartan |
|---|
| SMILES | CC1=NC(C)=C2CCC(=O)N(CC3=CC=C(C=C3)C3=CC=CC=C3C3=NNN=N3)C2=N1 |
|---|
| InChI Identifier | InChI=1S/C23H21N7O/c1-14-18-11-12-21(31)30(23(18)25-15(2)24-14)13-16-7-9-17(10-8-16)19-5-3-4-6-20(19)22-26-28-29-27-22/h3-10H,11-13H2,1-2H3,(H,26,27,28,29) |
|---|
| InChI Key | ADXGNEYLLLSOAR-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as biphenyls and derivatives. These are organic compounds containing to benzene rings linked together by a C-C bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Biphenyls and derivatives |
|---|
| Direct Parent | Biphenyls and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Biphenyl
- Phenyltetrazole
- Pyridopyrimidine
- Pyridine
- Pyrimidine
- Imidolactam
- Azole
- Tertiary carboxylic acid amide
- Tetrazole
- Heteroaromatic compound
- Lactam
- Carboxamide group
- Carboxylic acid derivative
- Organoheterocyclic compound
- Azacycle
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Carbonyl group
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001i-0549000000-19495bd41ec12908ff70 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0131900000-f3af74bdd6629a105726 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03dr-0879300000-8269237632c039f12e52 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-1592000000-980b45c1d09131ebdf6e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0400900000-fd81ab030f2eb998e74b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01t9-0923500000-5cca310860df9ec45c87 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0nov-1900000000-f6721900e79fe657f4f2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0000900000-f6647abdaaca45ff0208 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0bti-0097300000-d053fab47bdde20f7cc4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0pdi-0729000000-ea79016bd9dcefdca2df | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0000900000-71807d3326e25477a37c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0i2c-4109300000-5ac40be4ec7700a0bb47 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-02mi-1922000000-805b0ac35d44bed29861 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01349 |
|---|
| HMDB ID | HMDB0015439 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Tasosartan |
|---|
| Chemspider ID | 54890 |
|---|
| ChEBI ID | 215967 |
|---|
| PubChem Compound ID | 60919 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|