| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:27:01 UTC |
|---|
| Update Date | 2016-11-09 01:17:27 UTC |
|---|
| Accession Number | CHEM022290 |
|---|
| Identification |
|---|
| Common Name | Saprisartan |
|---|
| Class | Small Molecule |
|---|
| Description | Saprisartan is an AT1 receptor antagonist. It is based on medications of losartan's prototypical chemical structure. The mode of (functional) AT1 receptor antagonism has been characterized as insurmountable/noncompetitive for saprisartan. It is very likely that slow dissociation kinetics from the AT1 receptor underlie insurmountable antagonism [A14009]. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
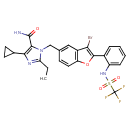
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| GR-138950C | HMDB | | GR-138950SAprisartan | HMDB | | Saprisartan potassium | HMDB | | Sapri-sartan potassium | HMDB | | 1-({3-bromo-2-[2-(trifluoromethanesulfonamido)phenyl]-1-benzofuran-5-yl}methyl)-4-cyclopropyl-2-ethyl-1H-imidazole-5-carboximidate | HMDB | | 1-({3-bromo-2-[2-(trifluoromethanesulphonamido)phenyl]-1-benzofuran-5-yl}methyl)-4-cyclopropyl-2-ethyl-1H-imidazole-5-carboximidate | HMDB | | 1-({3-bromo-2-[2-(trifluoromethanesulphonamido)phenyl]-1-benzofuran-5-yl}methyl)-4-cyclopropyl-2-ethyl-1H-imidazole-5-carboximidic acid | HMDB |
|
|---|
| Chemical Formula | C25H22BrF3N4O4S |
|---|
| Average Molecular Mass | 611.431 g/mol |
|---|
| Monoisotopic Mass | 610.050 g/mol |
|---|
| CAS Registry Number | 146623-69-0 |
|---|
| IUPAC Name | 1-{[3-bromo-2-(2-trifluoromethanesulfonamidophenyl)-1-benzofuran-5-yl]methyl}-4-cyclopropyl-2-ethyl-1H-imidazole-5-carboxamide |
|---|
| Traditional Name | saprisartan |
|---|
| SMILES | CCC1=NC(C2CC2)=C(N1CC1=CC2=C(OC(=C2Br)C2=CC=CC=C2NS(=O)(=O)C(F)(F)F)C=C1)C(N)=O |
|---|
| InChI Identifier | InChI=1S/C25H22BrF3N4O4S/c1-2-19-31-21(14-8-9-14)22(24(30)34)33(19)12-13-7-10-18-16(11-13)20(26)23(37-18)15-5-3-4-6-17(15)32-38(35,36)25(27,28)29/h3-7,10-11,14,32H,2,8-9,12H2,1H3,(H2,30,34) |
|---|
| InChI Key | DUEWVPTZCSAMNB-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 2-arylbenzofuran flavonoids. These are phenylpropanoids containing the 2-phenylbenzofuran moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | 2-arylbenzofuran flavonoids |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | 2-arylbenzofuran flavonoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - 2-arylbenzofuran flavonoid
- 2-phenylbenzofuran
- Phenylbenzofuran
- Sulfanilide
- 1,2,4,5-tetrasubstituted imidazole
- Benzofuran
- 2-heteroaryl carboxamide
- Imidazole-4-carbonyl group
- Aryl bromide
- Aryl halide
- Monocyclic benzene moiety
- N-substituted imidazole
- Organic sulfonic acid amide
- Benzenoid
- Organosulfonic acid amide
- Sulfonyl
- Aminosulfonyl compound
- Organosulfonic acid or derivatives
- Imidazole
- Organic sulfonic acid or derivatives
- Furan
- Heteroaromatic compound
- Azole
- Trihalomethane
- Carboxamide group
- Primary carboxylic acid amide
- Oxacycle
- Carboxylic acid derivative
- Azacycle
- Organoheterocyclic compound
- Organosulfur compound
- Alkyl fluoride
- Organohalogen compound
- Organobromide
- Organic oxygen compound
- Organofluoride
- Hydrocarbon derivative
- Halomethane
- Organic nitrogen compound
- Organic oxide
- Organopnictogen compound
- Alkyl halide
- Organonitrogen compound
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0lea-6500390000-e400299fe00d75260a2e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0110957000-a72ae38c2d41a4d60cf1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0000900000-e332f5eb30820cdefa1b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0ue9-9021500000-d8d9f1041ce1d4137815 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0730089000-195bd95c5056ce93a63f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-2900030000-9282f8ed9c687585fb9a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-2900000000-5f1e68080baaa3c60966 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03dl-0000049000-098806be38c74fe6e127 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03dl-0500193000-b2db80631faac397478e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0019-0911550000-4a7fe929cb900032d68d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0100009000-b6ab27fdb52b44c0b1c0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9000000000-2255eda51b844d32f5ee | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004u-6900341000-ddff270a833fd4e01ed8 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01347 |
|---|
| HMDB ID | HMDB0015437 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 54892 |
|---|
| ChEBI ID | 198341 |
|---|
| PubChem Compound ID | 60921 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|