| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:26:36 UTC |
|---|
| Update Date | 2016-11-09 01:17:27 UTC |
|---|
| Accession Number | CHEM022278 |
|---|
| Identification |
|---|
| Common Name | Pirbuterol |
|---|
| Class | Small Molecule |
|---|
| Description | Pirbuterol is a beta-2 adrenergic bronchodilator. In vitro studies and in vivo pharmacologic studies have demonstrated that pirbuterol has a preferential effect on beta-2 Adrenergic receptors compared with isoproterenol. While it is recognized that beta-2 adrenergic receptors are the predominant receptors in bronchial smooth muscle, data indicate that there is a population of beta-2 receptors in the human heart, existing in a concentration between 10-50%. The precise function of these receptors has not been established.
The pharmacologic effects of beta adrenergic agonist drugs, including pirbuterol, are at least in proof attributable to stimulation through beta adrenergic receptors of intracellular adenyl cyclase, the enzyme which catalyzes the conversion of adenosine triphosphate (AlP) to cyclic-3† ,5†-adenosine monophosphate (c-AMP). Increased c-AMP levels are associated with relaxation of bronchial smooth muscle and inhibition of release of mediators of immediate hypersensitivity from cells, especially from mast cells. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
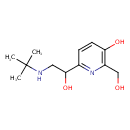
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3m Brand OF pirbuterol acetate | HMDB | | 2-Hydroxymethyl-3-hydroxy-6-(1-hydroxy-2-tert-butylamino ethyl)pyridine, dihydrochloride | HMDB | | Pyrbuterol | HMDB | | Maxair | HMDB | | Pirbuterol acetate salt | HMDB | | Pirbuterol sulfate | HMDB | | Pirbuterol acetate | HMDB | | Pirbuterol dihydrochloride | HMDB |
|
|---|
| Chemical Formula | C12H20N2O3 |
|---|
| Average Molecular Mass | 240.299 g/mol |
|---|
| Monoisotopic Mass | 240.147 g/mol |
|---|
| CAS Registry Number | 38677-81-5 |
|---|
| IUPAC Name | 6-[2-(tert-butylamino)-1-hydroxyethyl]-2-(hydroxymethyl)pyridin-3-ol |
|---|
| Traditional Name | pirbuterol |
|---|
| SMILES | CC(C)(C)NCC(O)C1=NC(CO)=C(O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C12H20N2O3/c1-12(2,3)13-6-11(17)8-4-5-10(16)9(7-15)14-8/h4-5,11,13,15-17H,6-7H2,1-3H3 |
|---|
| InChI Key | VQDBNKDJNJQRDG-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as hydroxypyridines. These are organic compounds containing a pyridine ring substituted at one or more positions by a hydroxyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pyridines and derivatives |
|---|
| Sub Class | Hydroxypyridines |
|---|
| Direct Parent | Hydroxypyridines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aralkylamine
- Hydroxypyridine
- Heteroaromatic compound
- 1,2-aminoalcohol
- Secondary alcohol
- Secondary aliphatic amine
- Secondary amine
- Azacycle
- Aromatic alcohol
- Alcohol
- Organic oxygen compound
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Organopnictogen compound
- Amine
- Hydrocarbon derivative
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0zn9-8930000000-28ba1967222e8cc4819f | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-059w-6596400000-849ea0d48532e8377ab9 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00dl-0290000000-ab8a2373770f64a2230e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01bi-2960000000-9d3af801ef3d4675039c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05g0-9610000000-ea7d70a1ef526d598c8f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-1190000000-3b6cc63af98c032e2be4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00dr-6690000000-9c6bc55993f92e172353 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00dl-9500000000-9deb72d565449648c680 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0290000000-ecd2bf5f75b39e8ea88e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-066s-3910000000-9acc70057d44ac07022e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9300000000-eea2f5657e650edf7f06 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0079-0090000000-912089ed17b08382a2fa | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0590000000-cdc26d7f311524f56d9c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-8900000000-917c7ae7f45779331092 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01291 |
|---|
| HMDB ID | HMDB0015407 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Pirbuterol |
|---|
| Chemspider ID | 4679 |
|---|
| ChEBI ID | 724172 |
|---|
| PubChem Compound ID | 4845 |
|---|
| Kegg Compound ID | C07807 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|