| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:26:27 UTC |
|---|
| Update Date | 2016-11-09 01:17:27 UTC |
|---|
| Accession Number | CHEM022273 |
|---|
| Identification |
|---|
| Common Name | Sunitinib |
|---|
| Class | Small Molecule |
|---|
| Description | Sunitinib is a small-molecule multi-targeted receptor tyrosine kinase (RTK) inhibitor. On January 26, 2006, the agent was formally approved by the US FDA for the indications of treating renal cell carcinoma (RCC) and imatinib-resistant gastrointestinal stromal tumor (GIST). For these purposes, sunitinib is generally available as an orally administered formulation. Sunitinib inhibits cellular signaling by targeting multiple RTKs. These include all platelet-derived growth factor receptors (PDGF-R) and vascular endothelial growth factor receptors (VEGF-R). Sunitinib also inhibits KIT (CD117), the RTK that drives the majority of GISTs. In addition, sunitinib inhibits other RTKs including RET, CSF-1R, and flt3. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
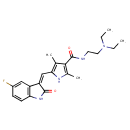
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| SU-11248 | ChEBI | | Sunitinibum | ChEBI | | Sutent | ChEBI | | SU11248 | HMDB | | Sunitinib malate | HMDB | | 5-(5-Fluoro-2-oxo-1,2-dihydroindolylidenemethyl)-2,4-dimethyl-1H-pyrrole-3-carboxylic acid (2-diethylaminoethyl)amide | HMDB |
|
|---|
| Chemical Formula | C22H27FN4O2 |
|---|
| Average Molecular Mass | 398.474 g/mol |
|---|
| Monoisotopic Mass | 398.212 g/mol |
|---|
| CAS Registry Number | 557795-19-4 |
|---|
| IUPAC Name | N-[2-(diethylamino)ethyl]-5-{[(3Z)-5-fluoro-2-oxo-2,3-dihydro-1H-indol-3-ylidene]methyl}-2,4-dimethyl-1H-pyrrole-3-carboxamide |
|---|
| Traditional Name | sunitinib |
|---|
| SMILES | CCN(CC)CCNC(=O)C1=C(C)NC(\C=C2/C(=O)NC3=C2C=C(F)C=C3)=C1C |
|---|
| InChI Identifier | InChI=1S/C22H27FN4O2/c1-5-27(6-2)10-9-24-22(29)20-13(3)19(25-14(20)4)12-17-16-11-15(23)7-8-18(16)26-21(17)28/h7-8,11-12,25H,5-6,9-10H2,1-4H3,(H,24,29)(H,26,28)/b17-12- |
|---|
| InChI Key | WINHZLLDWRZWRT-ATVHPVEESA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as indolines. Indolines are compounds containing an indole moiety, which consists of pyrrolidine ring fused to benzene to form 2,3-dihydroindole. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Indoles and derivatives |
|---|
| Sub Class | Indolines |
|---|
| Direct Parent | Indolines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dihydroindole
- Pyrrole-3-carboxamide
- Pyrrole-3-carboxylic acid or derivatives
- Aryl fluoride
- Aryl halide
- Substituted pyrrole
- Benzenoid
- Pyrrole
- Heteroaromatic compound
- Vinylogous amide
- Tertiary amine
- Amino acid or derivatives
- Secondary carboxylic acid amide
- Tertiary aliphatic amine
- Carboxamide group
- Lactam
- Azacycle
- Carboxylic acid derivative
- Amine
- Organofluoride
- Organohalogen compound
- Organonitrogen compound
- Organopnictogen compound
- Organooxygen compound
- Organic oxide
- Carbonyl group
- Organic oxygen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001r-9043000000-f9288d8cb7c447b10d86 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0419000000-d91569989d4c1e438a58 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-1039-4964000000-32cdbd9b3679f4b806d9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-9740000000-14530852543a6132d98b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0019000000-716107e6148bcb3af14c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052b-5398000000-e20fbe1cd3f11ad37acb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9230000000-0d8caf257003c837c3d6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0009000000-c7521df282758983a81b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-003r-0197000000-fc31f5a3f60673e3fb48 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-0293000000-c9aae10759016725c932 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0009000000-f6f1dd2f48935333770c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-2049000000-fe9e71e910a9eddb2023 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052g-6194000000-ce0d447fabdb293e1813 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01268 |
|---|
| HMDB ID | HMDB0015397 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | B49 |
|---|
| Wikipedia Link | Sunitinib |
|---|
| Chemspider ID | 4486264 |
|---|
| ChEBI ID | 38940 |
|---|
| PubChem Compound ID | 5329102 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, McArthur G, Judson IR, Heinrich MC, Morgan JA, Desai J, Fletcher CD, George S, Bello CL, Huang X, Baum CM, Casali PG: Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: a randomised controlled trial. Lancet. 2006 Oct 14;368(9544):1329-38. | | 2. Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, Oudard S, Negrier S, Szczylik C, Kim ST, Chen I, Bycott PW, Baum CM, Figlin RA: Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007 Jan 11;356(2):115-24. | | 3. FDA label | | 4. https://www.ncbi.nlm.nih.gov/pubmed/?term=12531805 | | 5. https://www.ncbi.nlm.nih.gov/pubmed/?term=16845442 | | 6. https://www.ncbi.nlm.nih.gov/pubmed/?term=16916320 | | 7. https://www.ncbi.nlm.nih.gov/pubmed/?term=17327610 | | 8. https://www.ncbi.nlm.nih.gov/pubmed/?term=17962201 | | 9. https://www.ncbi.nlm.nih.gov/pubmed/?term=18971320 | | 10. https://www.ncbi.nlm.nih.gov/pubmed/?term=19830602 | | 11. https://www.ncbi.nlm.nih.gov/pubmed/?term=20406969 | | 12. https://www.ncbi.nlm.nih.gov/pubmed/?term=21792888 | | 13. https://www.ncbi.nlm.nih.gov/pubmed/?term=24188025 | | 14. https://www.ncbi.nlm.nih.gov/pubmed/?term=24393200 | | 15. https://www.ncbi.nlm.nih.gov/pubmed/?term=24402960 | | 16. https://www.ncbi.nlm.nih.gov/pubmed/?term=24403097 | | 17. https://www.ncbi.nlm.nih.gov/pubmed/?term=24521256 |
|
|---|