| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:26:19 UTC |
|---|
| Update Date | 2016-11-09 01:17:27 UTC |
|---|
| Accession Number | CHEM022269 |
|---|
| Identification |
|---|
| Common Name | Lisdexamfetamine |
|---|
| Class | Small Molecule |
|---|
| Description | Also known as _Vyvanse_, lisdexamfetamine (L-lysine-d-amphetamine) is a prodrug of the psychostimulant d-amphetamine [A40246]. It is paired with the essential amino acid _L-lysine_. Lisdexamfetamine dimesylate increases attention span and decreases restlessness in children and adults who are overactive/hyperactive, cannot concentrate for long periods, or are easily distracted or impulsive [A2230].
As a central nervous system stimulant, lisdexamfetamine is utilized as an adjunct therapy in the treatment of attention deficit hyperactivity disorder (ADHD). As a prodrug, lisdexamfetamine was specifically engineered as an abuse-resistant product [F2368]. The mechanism by which this occurs is through delayed release after ingestion (unlike some other psychostimulant drugs, which may be abused). After oral administration and absorption, enzyme hydrolysis after contact with red blood cells metabolize lisdexamfetamine into L- lysine, a naturally occurring essential amino acid and active _d-amphetamine_, which is responsible for the drug’s pharmacological effects. Gastrointestinal pH does not affect this conversion, and the addition of the L-lysine slows the amount of d-amphetamine available in the circulation and central nervous system [F2368]. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
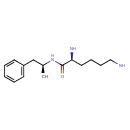
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Lisdexamfetamine dimesylate | HMDB | | NRP104 | HMDB | | Vyvanse | HMDB | | Lis dexamfetamine dimesylate | HMDB | | NRP 104 | HMDB | | Elvanse | HMDB | | Lis-dexamfetamine dimesylate | HMDB | | Dimesylate, lis-dexamfetamine | HMDB | | Dimesylate, lisdexamfetamine | HMDB | | NRP-104 | HMDB |
|
|---|
| Chemical Formula | C15H25N3O |
|---|
| Average Molecular Mass | 263.379 g/mol |
|---|
| Monoisotopic Mass | 263.200 g/mol |
|---|
| CAS Registry Number | 608137-32-2 |
|---|
| IUPAC Name | (2S)-2,6-diamino-N-[(2S)-1-phenylpropan-2-yl]hexanamide |
|---|
| Traditional Name | vyvanse |
|---|
| SMILES | C[C@@H](CC1=CC=CC=C1)NC(=O)[C@@H](N)CCCCN |
|---|
| InChI Identifier | InChI=1S/C15H25N3O/c1-12(11-13-7-3-2-4-8-13)18-15(19)14(17)9-5-6-10-16/h2-4,7-8,12,14H,5-6,9-11,16-17H2,1H3,(H,18,19)/t12-,14-/m0/s1 |
|---|
| InChI Key | VOBHXZCDAVEXEY-JSGCOSHPSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as alpha amino acid amides. These are amide derivatives of alpha amino acids. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Alpha amino acid amides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-amino acid amide
- Amphetamine or derivatives
- Phenylpropane
- Monocyclic benzene moiety
- Fatty amide
- Fatty acyl
- Benzenoid
- N-acyl-amine
- Carboxamide group
- Secondary carboxylic acid amide
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Primary amine
- Primary aliphatic amine
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Organopnictogen compound
- Organic oxygen compound
- Amine
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0udi-7910000000-cf67fb00e86045c2e167 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01qa-1980000000-d55cfea0090eb8a203ec | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-3910000000-6c0e093306cb1a142d27 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00lr-9600000000-0319b363d14e7fb37bdb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0290000000-3d4c25de9940b1f84b65 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01sl-1940000000-934e79a23f532e705cd7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00lu-4900000000-c557594a41309e8f3655 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0090000000-378898bcf4a9421cb8d5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01ql-9780000000-45bb7d0848ffcb464b5b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9100000000-4e7bbad339ecdb03e6c4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0090000000-45f7796828e5f04a3c2f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01t9-6920000000-8183a8d18b1b49ca7221 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9100000000-7d80f02475e4b0ca64f3 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01255 |
|---|
| HMDB ID | HMDB0015385 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Lisdexamfetamine |
|---|
| Chemspider ID | 9772458 |
|---|
| ChEBI ID | 775173 |
|---|
| PubChem Compound ID | 11597698 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|