| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:25:47 UTC |
|---|
| Update Date | 2016-11-09 01:17:27 UTC |
|---|
| Accession Number | CHEM022256 |
|---|
| Identification |
|---|
| Common Name | Idarubicin |
|---|
| Class | Small Molecule |
|---|
| Description | An orally administered anthracycline antineoplastic. The compound has shown activity against breast cancer, lymphomas and leukemias, together with the potential for reduced cardiac toxicity. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
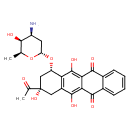
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (1S,3S)-3-Acetyl-3,5,12-trihydroxy-6,11-dioxo-1,2,3,4,6,11-hexahydronaphthacen-1-yl 3-amino-2,3,6-trideoxy-alpha-L-lyxo-hexopyranoside | ChEBI | | 4-Demethoxydaunomycin | ChEBI | | 4-Demethoxydaunorubicin | ChEBI | | Zavedos | Kegg | | (1S,3S)-3-Acetyl-3,5,12-trihydroxy-6,11-dioxo-1,2,3,4,6,11-hexahydronaphthacen-1-yl 3-amino-2,3,6-trideoxy-a-L-lyxo-hexopyranoside | Generator | | (1S,3S)-3-Acetyl-3,5,12-trihydroxy-6,11-dioxo-1,2,3,4,6,11-hexahydronaphthacen-1-yl 3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranoside | Generator | | 4 Desmethoxydaunorubicin | HMDB | | IMI30 | HMDB | | 4-Desmethoxydaunorubicin | HMDB | | Hydrochloride, idarubicin | HMDB | | 4 Demethoxydaunorubicin | HMDB | | IMI 30 | HMDB | | IMI-30 | HMDB | | Idarubicin hydrochloride | HMDB |
|
|---|
| Chemical Formula | C26H27NO9 |
|---|
| Average Molecular Mass | 497.494 g/mol |
|---|
| Monoisotopic Mass | 497.169 g/mol |
|---|
| CAS Registry Number | 58957-92-9 |
|---|
| IUPAC Name | (7S,9S)-9-acetyl-7-{[(2R,4S,5S,6S)-4-amino-5-hydroxy-6-methyloxan-2-yl]oxy}-6,9,11-trihydroxy-5,7,8,9,10,12-hexahydrotetracene-5,12-dione |
|---|
| Traditional Name | idarubicin |
|---|
| SMILES | C[C@@H]1O[C@H](C[C@H](N)[C@@H]1O)O[C@H]1C[C@@](O)(CC2=C1C(O)=C1C(=O)C3=CC=CC=C3C(=O)C1=C2O)C(C)=O |
|---|
| InChI Identifier | InChI=1S/C26H27NO9/c1-10-21(29)15(27)7-17(35-10)36-16-9-26(34,11(2)28)8-14-18(16)25(33)20-19(24(14)32)22(30)12-5-3-4-6-13(12)23(20)31/h3-6,10,15-17,21,29,32-34H,7-9,27H2,1-2H3/t10-,15-,16-,17-,21+,26-/m0/s1 |
|---|
| InChI Key | XDXDZDZNSLXDNA-TZNDIEGXSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as diphenylmethanes. Diphenylmethanes are compounds containing a diphenylmethane moiety, which consists of a methane wherein two hydrogen atoms are replaced by two phenyl groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Diphenylmethanes |
|---|
| Direct Parent | Diphenylmethanes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Diphenylmethane
- Aralkylamine
- N-alkylpiperazine
- N-methylpiperazine
- 1,4-diazinane
- Piperazine
- Tertiary amine
- Tertiary aliphatic amine
- Azacycle
- Organoheterocyclic compound
- Hydrocarbon derivative
- Organonitrogen compound
- Organopnictogen compound
- Organic nitrogen compound
- Amine
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9001300000-2de3fa2c7773861d551e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-004i-9201235000-1667e96cf86211584cde | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - , positive | splash10-000x-0393000000-ddc13e189dad3ac7e41a | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-000x-1593000000-69c216582ee995c4994a | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - 35V, Negative | splash10-0002-0009200000-bc576e831293830ef01e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0fsj-0007900000-f98135a681be27de52fd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uxr-0329100000-be91546e966302a56139 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-9257000000-7462490f63ce54df520d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-1104900000-b9f2288cd69935e73d74 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-016s-2109400000-e516d0132a62935de379 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-3119000000-f69c0c8721bcd3c2b66e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0008900000-85b3a0d465f7ec214bae | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001r-0409600000-cd3feeae87163d339d81 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-08gr-9632700000-6a3e9d575e1fa19c841d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0004900000-d3fce72bd7a4bc261905 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-0009000000-fd6d8e90c9f112c7cdd1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00ls-1009100000-00fb149725f785725656 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01177 |
|---|
| HMDB ID | HMDB0015308 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Idarubicin |
|---|
| Chemspider ID | 39117 |
|---|
| ChEBI ID | 42068 |
|---|
| PubChem Compound ID | 42890 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|