| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:25:21 UTC |
|---|
| Update Date | 2016-11-09 01:17:27 UTC |
|---|
| Accession Number | CHEM022244 |
|---|
| Identification |
|---|
| Common Name | Micafungin |
|---|
| Class | Small Molecule |
|---|
| Description | Micafungin is an antifungal drug. It belongs to the antifungal class of compounds known as echinocandins and exerts its effect by inhibiting the synthesis of 1,3-beta-D-glucan, an integral component of the fungal cell wall. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
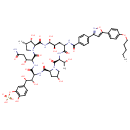
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Mycamine | ChEBI | | FK-463 | HMDB | | Micafungin sodium | HMDB |
|
|---|
| Chemical Formula | C56H71N9O23S |
|---|
| Average Molecular Mass | 1270.274 g/mol |
|---|
| Monoisotopic Mass | 1269.438 g/mol |
|---|
| CAS Registry Number | 235114-32-6 |
|---|
| IUPAC Name | {5-[(1S,2S)-2-[(3S,6S,9S,11R,15S,18S,20R,21R,24S,25S,26S)-3-[(1R)-2-carbamoyl-1-hydroxyethyl]-11,20,21,25-tetrahydroxy-15-[(1R)-1-hydroxyethyl]-26-methyl-2,5,8,14,17,23-hexaoxo-18-(4-{5-[4-(pentyloxy)phenyl]-1,2-oxazol-3-yl}benzamido)-1,4,7,13,16,22-hexaazatricyclo[22.3.0.0⁹,¹³]heptacosan-6-yl]-1,2-dihydroxyethyl]-2-hydroxyphenyl}oxidanesulfonic acid |
|---|
| Traditional Name | micafungin |
|---|
| SMILES | CCCCCOC1=CC=C(C=C1)C1=CC(=NO1)C1=CC=C(C=C1)C(=O)N[C@H]1C[C@@H](O)[C@@H](O)NC(=O)[C@@H]2[C@@H](O)[C@@H](C)CN2C(=O)[C@@H](NC(=O)[C@@H](NC(=O)[C@@H]2C[C@@H](O)CN2C(=O)[C@@H](NC1=O)[C@@H](C)O)[C@H](O)[C@@H](O)C1=CC(OS(O)(=O)=O)=C(O)C=C1)[C@H](O)CC(N)=O |
|---|
| InChI Identifier | InChI=1S/C56H71N9O23S/c1-4-5-6-17-86-32-14-11-28(12-15-32)39-21-33(63-87-39)27-7-9-29(10-8-27)49(75)58-34-20-38(70)52(78)62-54(80)45-46(72)25(2)23-65(45)56(82)43(37(69)22-41(57)71)60-53(79)44(48(74)47(73)30-13-16-36(68)40(18-30)88-89(83,84)85)61-51(77)35-19-31(67)24-64(35)55(81)42(26(3)66)59-50(34)76/h7-16,18,21,25-26,31,34-35,37-38,42-48,52,66-70,72-74,78H,4-6,17,19-20,22-24H2,1-3H3,(H2,57,71)(H,58,75)(H,59,76)(H,60,79)(H,61,77)(H,62,80)(H,83,84,85)/t25-,26+,31+,34-,35-,37+,38+,42-,43-,44-,45-,46-,47-,48-,52+/m0/s1 |
|---|
| InChI Key | PIEUQSKUWLMALL-YABMTYFHSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Oligopeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-oligopeptide
- Cyclic alpha peptide
- Macrolactam
- N-acyl-alpha amino acid or derivatives
- Phenylsulfate
- Alpha-amino acid or derivatives
- Arylsulfate
- Benzamide
- Benzoic acid or derivatives
- Phenoxy compound
- Benzoyl
- Phenol ether
- Alkyl aryl ether
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Monocyclic benzene moiety
- Fatty amide
- Fatty acyl
- Benzenoid
- Sulfuric acid monoester
- Sulfate-ester
- Sulfuric acid ester
- Heteroaromatic compound
- Isoxazole
- Organic sulfuric acid or derivatives
- Azole
- Tertiary carboxylic acid amide
- Pyrrolidine
- Secondary carboxylic acid amide
- Secondary alcohol
- Carboxamide group
- Primary carboxylic acid amide
- Lactam
- Ether
- Azacycle
- Oxacycle
- Alkanolamine
- Organoheterocyclic compound
- Polyol
- Organopnictogen compound
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Alcohol
- Organonitrogen compound
- Organooxygen compound
- Organic nitrogen compound
- Organic oxygen compound
- Aromatic alcohol
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f89-0090000000-4b64f397b40e9008eedd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-2291000000-4aa58ad76a6198021e14 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00e9-9453000001-3dc99a3a598f50ee7ca2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0fsi-0590000000-af7cc7a7d3742e6e3151 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-6970000000-2b09e28866be1a89cf60 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-015l-8940000003-178cb82ef6ea2cf80947 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0090000000-1a19fd3d6300f9b33adb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0kmi-1196000000-f2edf39e533b563fa71a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fdo-8921000008-d7d7832ba7a2829ff46a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0090000000-734ebe6b07172472a082 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-02t9-4690000001-fa2fc98848c5526a3370 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9230000001-ae99dc8d18ace78fb005 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB01141 |
|---|
| HMDB ID | HMDB0015272 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Micafungin |
|---|
| Chemspider ID | 419105 |
|---|
| ChEBI ID | 600520 |
|---|
| PubChem Compound ID | 477468 |
|---|
| Kegg Compound ID | C15819 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Groll AH, Stergiopoulou T, Roilides E, Walsh TJ: Micafungin: pharmacology, experimental therapeutics and clinical applications. Expert Opin Investig Drugs. 2005 Apr;14(4):489-509. | | 2. Chandrasekar PH, Sobel JD: Micafungin: a new echinocandin. Clin Infect Dis. 2006 Apr 15;42(8):1171-8. Epub 2006 Mar 14. | | 3. Morris MI, Villmann M: Echinocandins in the management of invasive fungal infections, part 1. Am J Health Syst Pharm. 2006 Sep 15;63(18):1693-703. | | 4. Morris MI, Villmann M: Echinocandins in the management of invasive fungal infections, Part 2. Am J Health Syst Pharm. 2006 Oct 1;63(19):1813-20. | | 5. Ikeda F, Tanaka S, Ohki H, Matsumoto S, Maki K, Katashima M, Barrett D, Aoki Y: Role of micafungin in the antifungal armamentarium. Curr Med Chem. 2007;14(11):1263-75. | | 6. Wiederhold NP, Lewis JS 2nd: The echinocandin micafungin: a review of the pharmacology, spectrum of activity, clinical efficacy and safety. Expert Opin Pharmacother. 2007 Jun;8(8):1155-66. | | 7. Vehreschild JJ, Cornely OA: Micafungin sodium, the second of the echinocandin class of antifungals: theory and practice. Future Microbiol. 2006 Aug;1(2):161-70. | | 8. Sucher AJ, Chahine EB, Balcer HE: Echinocandins: the newest class of antifungals. Ann Pharmacother. 2009 Oct;43(10):1647-57. doi: 10.1345/aph.1M237. Epub 2009 Sep 1. | | 9. Bormann AM, Morrison VA: Review of the pharmacology and clinical studies of micafungin. Drug Des Devel Ther. 2009 Dec 29;3:295-302. | | 10. Grover ND: Echinocandins: A ray of hope in antifungal drug therapy. Indian J Pharmacol. 2010 Feb;42(1):9-11. doi: 10.4103/0253-7613.62396. | | 11. https://www.ncbi.nlm.nih.gov/pubmed/?term=17194830 | | 12. https://www.ncbi.nlm.nih.gov/pubmed/?term=17307974 | | 13. https://www.ncbi.nlm.nih.gov/pubmed/?term=17325217 | | 14. https://www.ncbi.nlm.nih.gov/pubmed/?term=17325225 | | 15. https://www.ncbi.nlm.nih.gov/pubmed/?term=17420211 | | 16. https://www.ncbi.nlm.nih.gov/pubmed/?term=17785512 |
|
|---|