| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:24:28 UTC |
|---|
| Update Date | 2016-11-09 01:17:27 UTC |
|---|
| Accession Number | CHEM022220 |
|---|
| Identification |
|---|
| Common Name | Hydroxystilbamidine Isethionate |
|---|
| Class | Small Molecule |
|---|
| Description | Not Available |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
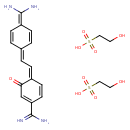
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (4Z)-4-{2-[4-(diaminomethylidene)cyclohexa-2,5-dien-1-ylidene]ethylidene}-3-oxocyclohexa-1,5-diene-1-carboximidamide; bis(2-hydroxyethane-1-sulfonate) | Generator | | (4Z)-4-{2-[4-(diaminomethylidene)cyclohexa-2,5-dien-1-ylidene]ethylidene}-3-oxocyclohexa-1,5-diene-1-carboximidamide; bis(2-hydroxyethane-1-sulphonate) | Generator | | (4Z)-4-{2-[4-(diaminomethylidene)cyclohexa-2,5-dien-1-ylidene]ethylidene}-3-oxocyclohexa-1,5-diene-1-carboximidamide; bis(2-hydroxyethane-1-sulphonic acid) | Generator | | HSB | HMDB | | 2-Hydroxystilbamidine isethionate | MeSH | | (4Z)-4-{2-[4-(diaminomethylidene)cyclohexa-2,5-dien-1-ylidene]ethylidene}-3-oxocyclohexa-1,5-diene-1-carboximidamide | | | bis(2-hydroxyethane-1-sulfonate) | | | bis(2-hydroxyethane-1-sulphonate) | | | bis(2-hydroxyethane-1-sulphonic acid) | |
|
|---|
| Chemical Formula | C20H28N4O9S2 |
|---|
| Average Molecular Mass | 532.588 g/mol |
|---|
| Monoisotopic Mass | 532.130 g/mol |
|---|
| CAS Registry Number | 533-22-2 |
|---|
| IUPAC Name | (4Z)-4-{2-[4-(diaminomethylidene)cyclohexa-2,5-dien-1-ylidene]ethylidene}-3-oxocyclohexa-1,5-diene-1-carboximidamide; bis(2-hydroxyethane-1-sulfonic acid) |
|---|
| Traditional Name | (4Z)-4-{2-[4-(diaminomethylidene)cyclohexa-2,5-dien-1-ylidene]ethylidene}-3-oxocyclohexa-1,5-diene-1-carboximidamide; bis(sodium isethionate) |
|---|
| SMILES | OCCS(O)(=O)=O.OCCS(O)(=O)=O.NC(N)=C1C=CC(=C\C=C2\C=CC(=CC2=O)C(N)=N)C=C1 |
|---|
| InChI Identifier | InChI=1S/C16H16N4O.2C2H6O4S/c17-15(18)12-5-2-10(3-6-12)1-4-11-7-8-13(16(19)20)9-14(11)21;2*3-1-2-7(4,5)6/h1-9H,17-18H2,(H3,19,20);2*3H,1-2H2,(H,4,5,6)/b11-4-;; |
|---|
| InChI Key | VIDDZUWWPMDEHU-LTTFOTGMSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as stilbenes. These are organic compounds containing a 1,2-diphenylethylene moiety. Stilbenes (C6-C2-C6 ) are derived from the common phenylpropene (C6-C3) skeleton building block. The introduction of one or more hydroxyl groups to a phenyl ring lead to stilbenoids. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Stilbenes |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Stilbenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Stilbene
- O-quinomethane
- P-quinodimethane
- Quinomethane
- Monocyclic benzene moiety
- Benzenoid
- Organic sulfonic acid or derivatives
- Organosulfonic acid or derivatives
- Organosulfonic acid
- Sulfonyl
- Alkanesulfonic acid
- Cyclic ketone
- Ketone
- Amidine
- Carboximidamide
- Carboxylic acid amidine
- Alcohol
- Organopnictogen compound
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Primary aliphatic amine
- Primary alcohol
- Organic oxide
- Organic nitrogen compound
- Hydrocarbon derivative
- Carbonyl group
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Not Available |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ( TMS) - 70eV, Positive | splash10-00dr-9167000000-2b97d22f283f7ce19c83 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0000090000-bca1934945063ef90875 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0000090000-bca1934945063ef90875 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-0000090000-bca1934945063ef90875 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0000090000-2dc383f22afc9d65d9a3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0000090000-2dc383f22afc9d65d9a3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-001i-0000090000-2dc383f22afc9d65d9a3 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0015174 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 16051924 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|