| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:24:05 UTC |
|---|
| Update Date | 2016-11-09 01:17:27 UTC |
|---|
| Accession Number | CHEM022212 |
|---|
| Identification |
|---|
| Common Name | Lomefloxacin |
|---|
| Class | Small Molecule |
|---|
| Description | Lomefloxacin is a fluoroquinolone antibiotic, used to treat bacterial infections including bronchitis and urinary tract infections (UTIs). Additionally, it has been employed for the prophylaxis of UTIs prior to surgery as well. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
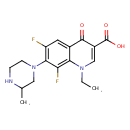
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+-)-1-Ethyl-6,8-difluoro-1,4-dihydro-7-(3-methyl-1-piperazinyl)-4-oxo-3-quinolinecarboxylic acid | ChEBI | | 1,4-Dihydro-6,8-difluoro-1-ethyl-7-(3-methyl-1-piperazinyl)-4-oxo-3-quinolinecarboxylic acid | ChEBI | | LFLX | ChEBI | | Lomefloxacine | ChEBI | | Lomefloxacino | ChEBI | | Lomefloxacinum | ChEBI | | (+-)-1-Ethyl-6,8-difluoro-1,4-dihydro-7-(3-methyl-1-piperazinyl)-4-oxo-3-quinolinecarboxylate | Generator | | 1,4-Dihydro-6,8-difluoro-1-ethyl-7-(3-methyl-1-piperazinyl)-4-oxo-3-quinolinecarboxylate | Generator | | Lomefloxacin hydrochloride | HMDB | | 1-Ethyl-6,8-difluoro-1,4-dihydro-7-(3-methyl-1-piperazinyl)-4-oxo-3-quinolinecarboxylic acid | HMDB | | Ocacin | HMDB | | Décalogiflox | HMDB | | Logiflox | HMDB | | Okacin | HMDB | | Lomenfloxacin | HMDB | | Okacyn | HMDB | | Pharmacia brand 1 OF lomefloxacin hydrochloride | HMDB | | Maxaquin | HMDB | | Pharmacia brand 2 OF lomefloxacin hydrochloride | HMDB |
|
|---|
| Chemical Formula | C17H19F2N3O3 |
|---|
| Average Molecular Mass | 351.348 g/mol |
|---|
| Monoisotopic Mass | 351.139 g/mol |
|---|
| CAS Registry Number | 98079-51-7 |
|---|
| IUPAC Name | 1-ethyl-6,8-difluoro-7-(3-methylpiperazin-1-yl)-4-oxo-1,4-dihydroquinoline-3-carboxylic acid |
|---|
| Traditional Name | lomefloxacin |
|---|
| SMILES | CCN1C=C(C(O)=O)C(=O)C2=CC(F)=C(N3CCNC(C)C3)C(F)=C12 |
|---|
| InChI Identifier | InChI=1S/C17H19F2N3O3/c1-3-21-8-11(17(24)25)16(23)10-6-12(18)15(13(19)14(10)21)22-5-4-20-9(2)7-22/h6,8-9,20H,3-5,7H2,1-2H3,(H,24,25) |
|---|
| InChI Key | ZEKZLJVOYLTDKK-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as monobactams. Monobactams are compounds comprising beta-lactam ring is alone and not fused to another ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Lactams |
|---|
| Sub Class | Beta lactams |
|---|
| Direct Parent | Monobactams |
|---|
| Alternative Parents | |
|---|
| Substituents | - Monobactam
- 1-phenylazetidine
- 2-phenylazetidine
- 1-hydroxy-2-unsubstituted benzenoid
- Fluorobenzene
- Halobenzene
- Phenol
- Aryl fluoride
- Aryl halide
- Monocyclic benzene moiety
- Benzenoid
- Tertiary carboxylic acid amide
- Azetidine
- Carboxamide group
- Secondary alcohol
- Azacycle
- Carboxylic acid derivative
- Organonitrogen compound
- Organofluoride
- Organohalogen compound
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Organopnictogen compound
- Alcohol
- Organic oxygen compound
- Organooxygen compound
- Aromatic alcohol
- Organic nitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0076-0059000000-9a020873d71afda0852e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0a4i-2146900000-42b37b0f4d359398913c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0009000000-a43b3d54ac0a38633f6b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0pir-3029000000-16497a4c27f71f504961 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0zml-5090000000-69ee2ed1785a83a7efaf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0pb9-0009000000-f451e33ca1450f3c318c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4r-2089000000-c691d19f5e309e23e2d1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9040000000-8eea8088e8777a3cdacb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0009000000-7258981d354fc72fb9dd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0009000000-ee8d89a838e68a0a2cb1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-5059000000-f0e2d4b268337790a2af | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0zg0-0049000000-dfb7af6faa9a0d289aa7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0550-0094000000-c2059c294fb1be76130f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004r-0090000000-b404fb16e16898bd86b5 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00978 |
|---|
| HMDB ID | HMDB0015113 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Lomefloxacin |
|---|
| Chemspider ID | 3811 |
|---|
| ChEBI ID | 116278 |
|---|
| PubChem Compound ID | 3948 |
|---|
| Kegg Compound ID | C07078 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|