| Synonyms | | Value | Source |
|---|
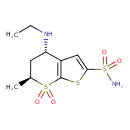
| (4S,6S)-4-Ethylamino-6-methyl-7,7-dioxo-4,5,6,7-tetrahydro-7lambda*6*-thieno[2,3-b]thiopyran-2-sulfonic acid amide | ChEBI | | (4S,trans)-4-(Ethylamino)-6-methyl-5,6-dihydro-4H-thieno[2,3-b]thiopyran-2-sulfonamide 7,7-dioxide | ChEBI | | (4S-trans)-4-(ETHYLAMINO)-5,6-dihydro-6-methyl-4H-thieno(2,3-b)thiopyran-2-sulfonamide-7,7-dioxide | ChEBI | | 4-Ethylamino-6-methyl-7,7-dioxo-4,5,6,7-tetrahydro-7lambda*6*-thieno[2,3-b]thiopyran-2-sulfonic acid amide | ChEBI | | 4-Ethylamino-6-methyl-7,7-dioxo-4,5,6,7-tetrahydro-7lambda6-thieno[2,3-b]thiopyran-2-sulfonic acid amide | ChEBI | | 4S,6S-Dorzolamide | ChEBI | | Trusopt | Kegg | | (4S,6S)-4-Ethylamino-6-methyl-7,7-dioxo-4,5,6,7-tetrahydro-7lambda*6*-thieno[2,3-b]thiopyran-2-sulfonate amide | Generator | | (4S,6S)-4-Ethylamino-6-methyl-7,7-dioxo-4,5,6,7-tetrahydro-7lambda*6*-thieno[2,3-b]thiopyran-2-sulphonate amide | Generator | | (4S,6S)-4-Ethylamino-6-methyl-7,7-dioxo-4,5,6,7-tetrahydro-7lambda*6*-thieno[2,3-b]thiopyran-2-sulphonic acid amide | Generator | | (4S,trans)-4-(Ethylamino)-6-methyl-5,6-dihydro-4H-thieno[2,3-b]thiopyran-2-sulphonamide 7,7-dioxide | Generator | | (4S-trans)-4-(ETHYLAMINO)-5,6-dihydro-6-methyl-4H-thieno(2,3-b)thiopyran-2-sulphonamide-7,7-dioxide | Generator | | 4-Ethylamino-6-methyl-7,7-dioxo-4,5,6,7-tetrahydro-7lambda*6*-thieno[2,3-b]thiopyran-2-sulfonate amide | Generator | | 4-Ethylamino-6-methyl-7,7-dioxo-4,5,6,7-tetrahydro-7lambda*6*-thieno[2,3-b]thiopyran-2-sulphonate amide | Generator | | 4-Ethylamino-6-methyl-7,7-dioxo-4,5,6,7-tetrahydro-7lambda*6*-thieno[2,3-b]thiopyran-2-sulphonic acid amide | Generator | | 4-Ethylamino-6-methyl-7,7-dioxo-4,5,6,7-tetrahydro-7lambda6-thieno[2,3-b]thiopyran-2-sulfonate amide | Generator | | 4-Ethylamino-6-methyl-7,7-dioxo-4,5,6,7-tetrahydro-7lambda6-thieno[2,3-b]thiopyran-2-sulphonate amide | Generator | | 4-Ethylamino-6-methyl-7,7-dioxo-4,5,6,7-tetrahydro-7lambda6-thieno[2,3-b]thiopyran-2-sulphonic acid amide | Generator | | 5,6-Dihydro-4-ethylamino-6-methyl-4H-thieno(2,3-b)thiopyran-2-sulfonamide-7,7-dioxide | HMDB | | Dorzolamide hydrochloride | HMDB | | Dorzolamide chibret | HMDB | | Dorzolamide, (trans)-isomer | HMDB | | 4-Ethylamino-5,6-dihydro-6-methyl-7,7-dioxide-4H-thieno(2,3-b)thiopyran-2-sulfonamide | HMDB |
|
|---|