| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:22:53 UTC |
|---|
| Update Date | 2016-11-09 01:17:26 UTC |
|---|
| Accession Number | CHEM022181 |
|---|
| Identification |
|---|
| Common Name | Tirofiban |
|---|
| Class | Small Molecule |
|---|
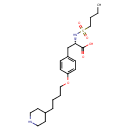
| Description | A member of the class of piperidines that is L-tyrosine in which a hydrogen attached to the amino group is replaced by a butylsulfonyl group and in which the hydrogen attached to the phenolic hydroxy group is replaced by a 4-(piperidin-4-yl)butyl group. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (2S)-2-(Butylsulfonylamino)-3-[4-(4-piperidin-4-ylbutoxy)phenyl]propanoic acid | ChEBI | | N-(Butylsulfonyl)-O-(4-(4-piperidyl)butyl)-L-tyrosine | ChEBI | | Tirofibanum | ChEBI | | Agrastat | Kegg | | (2S)-2-(Butylsulfonylamino)-3-[4-(4-piperidin-4-ylbutoxy)phenyl]propanoate | Generator | | (2S)-2-(Butylsulphonylamino)-3-[4-(4-piperidin-4-ylbutoxy)phenyl]propanoate | Generator | | (2S)-2-(Butylsulphonylamino)-3-[4-(4-piperidin-4-ylbutoxy)phenyl]propanoic acid | Generator | | N-(Butylsulphonyl)-O-(4-(4-piperidyl)butyl)-L-tyrosine | Generator | | Merck sharp and dohme brand OF tirofiban hydrochloride monohydrate | HMDB | | Tirofiban hydrochloride | HMDB | | Merck frosst brand OF tirofiban hydrochloride monohydrate | HMDB | | Merck brand OF tirofiban hydrochloride monohydrate | HMDB | | Tirofiban hydrochloride monohydrate | HMDB | | Aggrastat | HMDB | | Cahill may roberts brand OF tirofiban hydrochloride monohydrate | HMDB | | MSD Brand OF tirofiban hydrochloride monohydrate | HMDB |
|
|---|
| Chemical Formula | C22H36N2O5S |
|---|
| Average Molecular Mass | 440.597 g/mol |
|---|
| Monoisotopic Mass | 440.234 g/mol |
|---|
| CAS Registry Number | 144494-65-5 |
|---|
| IUPAC Name | (2S)-2-(butane-1-sulfonamido)-3-{4-[4-(piperidin-4-yl)butoxy]phenyl}propanoic acid |
|---|
| Traditional Name | tirofiban |
|---|
| SMILES | CCCCS(=O)(=O)N[C@@H](CC1=CC=C(OCCCCC2CCNCC2)C=C1)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C22H36N2O5S/c1-2-3-16-30(27,28)24-21(22(25)26)17-19-7-9-20(10-8-19)29-15-5-4-6-18-11-13-23-14-12-18/h7-10,18,21,23-24H,2-6,11-17H2,1H3,(H,25,26)/t21-/m0/s1 |
|---|
| InChI Key | COKMIXFXJJXBQG-NRFANRHFSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylalanine and derivatives. Phenylalanine and derivatives are compounds containing phenylalanine or a derivative thereof resulting from reaction of phenylalanine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Phenylalanine and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenylalanine or derivatives
- 3-phenylpropanoic-acid
- Amphetamine or derivatives
- Phenoxy compound
- Phenol ether
- Alkyl aryl ether
- Monocyclic benzene moiety
- Piperidine
- Benzenoid
- Organosulfonic acid amide
- Organic sulfonic acid amide
- Organic sulfonic acid or derivatives
- Aminosulfonyl compound
- Sulfonyl
- Organosulfonic acid or derivatives
- Amino acid
- Carboxylic acid
- Secondary aliphatic amine
- Ether
- Monocarboxylic acid or derivatives
- Azacycle
- Secondary amine
- Organoheterocyclic compound
- Organonitrogen compound
- Organic oxygen compound
- Amine
- Organooxygen compound
- Organosulfur compound
- Organic oxide
- Hydrocarbon derivative
- Organopnictogen compound
- Carbonyl group
- Organic nitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a4i-9233000000-449c9f26afd8b05e7422 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0a4j-9241200000-c8cce3f0150c49c7b4b9 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-006y-0549500000-2974f1cff243a2edea70 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-05fr-8978100000-c82b954a66a21d7b7b16 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-4960000000-99f01b2e16edac7fb882 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0417900000-2def9a867e11bd4431b8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-2947100000-e78108adb072e11a5add | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-024u-6940000000-5ebd43e473a27543a015 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0016900000-2cd3d85cb7f7753eceb7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-1096000000-d6ff280138c0c8f31cb8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-8910000000-a1aaa15fb128bd3ad5a3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00kr-1502900000-6b2945d27bec9c418749 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-9024000000-1371a4b5fb1f8011211d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-022a-9431100000-8c15bfa5f044a4504b3f | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00775 |
|---|
| HMDB ID | HMDB0014913 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Tirofiban |
|---|
| Chemspider ID | 54912 |
|---|
| ChEBI ID | 9605 |
|---|
| PubChem Compound ID | 60947 |
|---|
| Kegg Compound ID | C07965 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|