| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:22:46 UTC |
|---|
| Update Date | 2016-11-09 01:17:26 UTC |
|---|
| Accession Number | CHEM022177 |
|---|
| Identification |
|---|
| Common Name | Dolasetron |
|---|
| Class | Small Molecule |
|---|
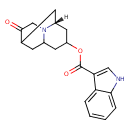
| Description | Dolasetron is an antinauseant and antiemetic agent indicated for the prevention of nausea and vomiting associated with moderately-emetogenic cancer chemotherapy and for the prevention of postoperative nausea and vomiting. Dolasetron is a highly specific and selective serotonin 5-HT3 receptor antagonist. This drug has not shown to have activity at other known serotonin receptors, and has low affinity for dopamine receptors. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Anzemet | HMDB | | MDL 73147Ef | HMDB | | MDL-73147Ef | HMDB | | MDL 73,147Ef | HMDB | | Octahydro-3-oxo-2,6-methano-2H-quinolizin-8-yl 1H-indole-3-carboxylate | HMDB | | Dolasetron mesylate | HMDB | | 1H-Indole-3-carboxylic acid-trans-octahydro-3-oxo-2,6-methano-2H-quinolizin-8-yl ester methanesulfonate | HMDB | | Dolasetron mesilate monohydrate | MeSH | | 1H-Indole-3-carboxylic acid, (6R,9as)-octahydro-3-oxo-2,6-methano-2H-quinolizin-8-yl ester, rel-, methanesulfonate, hydrate (1:1:1) | MeSH | | Dolasetron mesylate monohydrate | MeSH | | Indole-3-carboxylic acid, ester with (8R)-hexahydro-8-hydroxy-2,6-methano-2H-quinolizin-3(4H)-one | MeSH | | (3R)-10-oxo-8-Azatricyclo[5.3.1.0³,⁸]undecan-5-yl 1H-indole-3-carboxylic acid | Generator | | Dolasetron | MeSH | | (3R)-10-oxo-8-Azatricyclo[5.3.1.0,]undecan-5-yl 1H-indole-3-carboxylic acid | Generator |
|
|---|
| Chemical Formula | C19H20N2O3 |
|---|
| Average Molecular Mass | 324.374 g/mol |
|---|
| Monoisotopic Mass | 324.147 g/mol |
|---|
| CAS Registry Number | 115956-12-2 |
|---|
| IUPAC Name | (3R)-10-oxo-8-azatricyclo[5.3.1.0³,⁸]undecan-5-yl 1H-indole-3-carboxylate |
|---|
| Traditional Name | dolasetron |
|---|
| SMILES | [H][C@]12CC(CC3CC(C1)C(=O)CN23)OC(=O)C1=CNC2=CC=CC=C12 |
|---|
| InChI Identifier | InChI=1S/C19H20N2O3/c22-18-10-21-12-5-11(18)6-13(21)8-14(7-12)24-19(23)16-9-20-17-4-2-1-3-15(16)17/h1-4,9,11-14,20H,5-8,10H2/t11?,12-,13?,14?/m1/s1 |
|---|
| InChI Key | UKTAZPQNNNJVKR-DBBXXEFVSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as indolecarboxylic acids and derivatives. Indolecarboxylic acids and derivatives are compounds containing a carboxylic acid group (or a derivative thereof) linked to an indole. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Indoles and derivatives |
|---|
| Sub Class | Indolecarboxylic acids and derivatives |
|---|
| Direct Parent | Indolecarboxylic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Indolecarboxylic acid derivative
- Quinolizidine
- Indole
- Quinuclidone
- Pyrrole-3-carboxylic acid or derivatives
- Quinuclidine
- Piperidinone
- Piperidine
- Substituted pyrrole
- Benzenoid
- Vinylogous amide
- Pyrrole
- Heteroaromatic compound
- Tertiary amine
- Tertiary aliphatic amine
- Carboxylic acid ester
- Ketone
- Amino acid or derivatives
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Azacycle
- Amine
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Organic oxygen compound
- Organic nitrogen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-006x-1900000000-8dcd9035e77addcb92c2 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0809000000-e543237d634e768bf190 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03dl-0901000000-d6386713d3df49c9837e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00l6-0900000000-0b1c6b04e96536805498 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0509000000-085a8f6a1ddf99d931fe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01b9-0903000000-3363bde3d5011e6d4a88 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-0900000000-49ee7a167d32200b182c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0009000000-9b54b47c162811e47733 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0409000000-dcf423a8abd27c382925 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014l-1900000000-b5bcc02270654ef46e8e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0009000000-ffc9bb102611a33bd213 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0809000000-00ad969880df21e26b60 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-0902000000-f4b49f170c3bb0df26cd | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0014895 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Dolasetron |
|---|
| Chemspider ID | 54666 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 60654 |
|---|
| Kegg Compound ID | C07866 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|