| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:22:45 UTC |
|---|
| Update Date | 2016-11-09 01:17:26 UTC |
|---|
| Accession Number | CHEM022176 |
|---|
| Identification |
|---|
| Common Name | Epinastine |
|---|
| Class | Small Molecule |
|---|
| Description | Epinastine is used for the prevention of itching associated with allergic conjunctivitis. It has a multi-action effect that inhibits the allergic response in 3 ways: 1. stabilizes mast cells by preventing mast cell degranulation to control the allergic response, 2. prevents histamine binding to both the H1- and H2-receptors to stop itching and provide lasting protection, and 3. prevents the release of proinflammatory chemical mediators from the blood vessel to halt progression of the allergic response. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
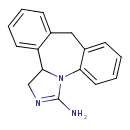
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+-)-Epinastine | ChEBI | | 3-Amino-9,13b-dihydro-1H-dibenz(c,F)imidazo(1,5-a)azepine | ChEBI | | Epinastina | ChEBI | | Epinastinum | ChEBI | | Purivist | Kegg | | WAL 801 CL | HMDB | | Flurinol | HMDB | | WAL 801 | HMDB | | 3-Amino-9,13b-dihydro-1H-benz(c,F)imidazo(1,5a)azepine | HMDB | | WAL 80 | HMDB | | WAL 801CL | HMDB | | WAL-80 CL | HMDB | | Epinastine hydrochloride | HMDB |
|
|---|
| Chemical Formula | C16H15N3 |
|---|
| Average Molecular Mass | 249.310 g/mol |
|---|
| Monoisotopic Mass | 249.127 g/mol |
|---|
| CAS Registry Number | 80012-43-7 |
|---|
| IUPAC Name | 2,4-diazatetracyclo[12.4.0.0²,⁶.0⁷,¹²]octadeca-1(18),3,7,9,11,14,16-heptaen-3-amine |
|---|
| Traditional Name | epinastine |
|---|
| SMILES | NC1=NCC2N1C1=CC=CC=C1CC1=CC=CC=C21 |
|---|
| InChI Identifier | InChI=1S/C16H15N3/c17-16-18-10-15-13-7-3-1-5-11(13)9-12-6-2-4-8-14(12)19(15)16/h1-8,15H,9-10H2,(H2,17,18) |
|---|
| InChI Key | WHWZLSFABNNENI-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dibenzazepines. Dibenzazepines are compounds with two benzene rings connected by an azepine ring. Azepine is an unsaturated seven-member heterocycle with one nitrogen atom replacing a carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Benzazepines |
|---|
| Sub Class | Dibenzazepines |
|---|
| Direct Parent | Dibenzazepines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dibenzazepine
- Azepine
- Benzenoid
- 2-imidazoline
- Guanidine
- Azacycle
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Carboximidamide
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00di-0590000000-b39e6b7f0c2561681ad3 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0190000000-55c54a74af25de5a147f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0190000000-2580dd8e371096c1da4a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-1910000000-f127e2e859edf1b82bbd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0090000000-02a99c90b5e69ce7b084 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-1090000000-d764b0b1b1062388fef5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-a161a19c732b38bb4e05 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0090000000-f0b3b54c94f5a6ad829d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0090000000-f0b3b54c94f5a6ad829d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0kgo-1390000000-f843e3127e653c3dda62 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0090000000-a6a269e69e0ce0f0732e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0090000000-e4df2bacabe4262b8e2e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9460000000-42957781cd670390aa21 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00751 |
|---|
| HMDB ID | HMDB0014889 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Epinastine |
|---|
| Chemspider ID | 3128 |
|---|
| ChEBI ID | 51032 |
|---|
| PubChem Compound ID | 3241 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|