| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:22:01 UTC |
|---|
| Update Date | 2016-11-09 01:17:26 UTC |
|---|
| Accession Number | CHEM022152 |
|---|
| Identification |
|---|
| Common Name | Gonadorelin |
|---|
| Class | Small Molecule |
|---|
| Description | Gonadorelin is another name for gonadotropin-releasing hormone (GnRH). It is a synthetic decapeptide prepared using solid phase peptide synthesis. GnRH is responsible for the release of follicle stimulating hormone and leutinizing hormone from the anterior pituitary. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
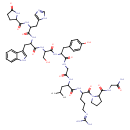
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Gonadoliberin I | Kegg | | GNRH-I | Kegg | | Fertagyl | Kegg | | Cystorelin | HMDB | | Gonadoliberin | HMDB | | Kryptocur | HMDB | | Luteinizing hormone-releasing hormone | HMDB | | FSH Releasing hormone | HMDB | | FSH-Releasing hormone | HMDB | | Factrel | HMDB | | Gonadorelin hydrochloride | HMDB | | lH-Releasing hormone | HMDB | | Gonadotropin releasing hormone | HMDB | | LHRH | HMDB | | Luteinizing hormone releasing hormone | HMDB | | lH-FSH Releasing hormone | HMDB | | LHFSHRH | HMDB | | GN-RH | HMDB | | Gonadotropin-releasing hormone | HMDB | | lH FSH Releasing hormone | HMDB | | lH Releasing hormone | HMDB | | Luliberin | HMDB | | Releasing hormone, LHFSH | HMDB | | GNRH | HMDB | | lH-RH | HMDB | | Gonadorelin acetate | HMDB | | LFRH | HMDB | | LHFSH Releasing hormone | HMDB | | Dirigestran | HMDB | | Gonadorelin | KEGG |
|
|---|
| Chemical Formula | C55H75N17O13 |
|---|
| Average Molecular Mass | 1182.290 g/mol |
|---|
| Monoisotopic Mass | 1181.573 g/mol |
|---|
| CAS Registry Number | 6918-09-8 |
|---|
| IUPAC Name | N-(1-{2-[(carbamoylmethyl)carbamoyl]pyrrolidin-1-yl}-5-[(diaminomethylidene)amino]-1-oxopentan-2-yl)-2-{2-[2-(3-hydroxy-2-{2-[3-(1H-imidazol-5-yl)-2-[(5-oxopyrrolidin-2-yl)formamido]propanamido]-3-(1H-indol-3-yl)propanamido}propanamido)-3-(4-hydroxyphenyl)propanamido]acetamido}-4-methylpentanamide |
|---|
| Traditional Name | gonadotropin releasing hormone |
|---|
| SMILES | CC(C)CC(NC(=O)CNC(=O)C(CC1=CC=C(O)C=C1)NC(=O)C(CO)NC(=O)C(CC1=CNC2=CC=CC=C12)NC(=O)C(CC1=CN=CN1)NC(=O)C1CCC(=O)N1)C(=O)NC(CCCN=C(N)N)C(=O)N1CCCC1C(=O)NCC(N)=O |
|---|
| InChI Identifier | InChI=1S/C55H75N17O13/c1-29(2)19-38(49(80)67-37(9-5-17-60-55(57)58)54(85)72-18-6-10-43(72)53(84)62-25-44(56)75)66-46(77)26-63-47(78)39(20-30-11-13-33(74)14-12-30)68-52(83)42(27-73)71-50(81)40(21-31-23-61-35-8-4-3-7-34(31)35)69-51(82)41(22-32-24-59-28-64-32)70-48(79)36-15-16-45(76)65-36/h3-4,7-8,11-14,23-24,28-29,36-43,61,73-74H,5-6,9-10,15-22,25-27H2,1-2H3,(H2,56,75)(H,59,64)(H,62,84)(H,63,78)(H,65,76)(H,66,77)(H,67,80)(H,68,83)(H,69,82)(H,70,79)(H,71,81)(H4,57,58,60) |
|---|
| InChI Key | XLXSAKCOAKORKW-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as polypeptides. These are peptides containing ten or more amino acid residues. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic Polymers |
|---|
| Class | Polypeptides |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Polypeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Polypeptide
- Alpha peptide
- Tyrosine or derivatives
- Phenylalanine or derivatives
- Histidine or derivatives
- Leucine or derivatives
- N-acyl-alpha amino acid or derivatives
- Proline or derivatives
- Triptan
- Alpha-amino acid amide
- Serine or derivatives
- N-substituted-alpha-amino acid
- Alpha-amino acid or derivatives
- Amphetamine or derivatives
- 3-alkylindole
- Indole
- Indole or derivatives
- N-acylpyrrolidine
- Pyrrolidine carboxylic acid or derivatives
- Pyrrolidine-2-carboxamide
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Monocyclic benzene moiety
- Fatty amide
- N-acyl-amine
- Fatty acyl
- Benzenoid
- Pyrrolidone
- 2-pyrrolidone
- Substituted pyrrole
- Imidazole
- Heteroaromatic compound
- Pyrrolidine
- Azole
- Tertiary carboxylic acid amide
- Pyrrole
- Guanidine
- Secondary carboxylic acid amide
- Carboxamide group
- Primary carboxylic acid amide
- Lactam
- Azacycle
- Carboxylic acid derivative
- Organoheterocyclic compound
- Organic 1,3-dipolar compound
- Carboximidamide
- Propargyl-type 1,3-dipolar organic compound
- Primary alcohol
- Organic nitrogen compound
- Hydrocarbon derivative
- Carbonyl group
- Organic oxide
- Organic oxygen compound
- Organopnictogen compound
- Organonitrogen compound
- Alcohol
- Organooxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | - Gonadotropin-releasing hormone [KO:K05252] (C07607 )
|
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-022a-9612402201-7dff9b7217a0eacedfb2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-9211300000-050a8099fd2f78a96960 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00e9-9010100000-8680e82fb261a184e63e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-08gi-2900100000-4512eaf4c4ccb20db288 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0c00-4900001000-7d38c37ce1a213415f70 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002f-9101002210-ee45e1d0424df22bb481 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-06sc-0601903210-931f1842aade74dcf605 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01ox-1420912010-f255f32c707911958961 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-0720912000-7a2d79ca06bf9d504e8d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001l-3901310000-81db4811a52fc0c2a20f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0c0c-8905528210-305e33d41cdb03e5ef1b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-007o-4910102210-6bd6f8d9e9a35a1f8595 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00644 |
|---|
| HMDB ID | HMDB0014782 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Gonadotropin-releasing hormone |
|---|
| Chemspider ID | 33562 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 36523 |
|---|
| Kegg Compound ID | C07607 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|