| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:21:58 UTC |
|---|
| Update Date | 2016-11-09 01:17:26 UTC |
|---|
| Accession Number | CHEM022151 |
|---|
| Identification |
|---|
| Common Name | Inulin |
|---|
| Class | Small Molecule |
|---|
| Description | Present in Jerusalem artichoke (Helianthus tuberosus) and in other members of Compositae
About 30?40% of people in Central Europe suffer from fructose malabsorption. Since inulin is a fructan, excess dietary intake may lead to minor side effects such as increased flatulence and loosened bowel motions in those with fructose malabsorption. It is recommended that fructan intake for people with fructose malabsorption be kept to less than 0.5 grams/serving.; For both inulin and creatinine, the calculations involve concentrations in the urine and in the serum. However, unlike creatinine, inulin is not naturally present in the body. This is an advantage of inulin (because the amount infused will be known) and a disadvantage (because an infusion is necessary.); Inulin is increasingly used in processed foods because it has unusually adaptable characteristics. Its flavour ranges from bland to subtly sweet (approx. 10% sweetness of sugar/sucrose). It can be used to replace sugar, fat, and flour. This is particularly advantageous because inulin contains a third to a quarter of the food energy of sugar or other carbohydrates and a sixth to a ninth of the food energy of fat. While Inulin is a versatile ingredient, it also has health benefits. Inulin increases calcium absorption and possibly magnesium absorption, while promoting the growth of intestinal bacteria. Nutritionally, it is considered a form of soluble fiber and is sometimes categorized as a prebiotic. Inulin has a minimal impact on blood sugar, and?unlike fructose?is not insulemic and does not raise triglycerides, making it generally considered suitable for diabetics and potentially helpful in managing blood sugar-related illnesses. The consumption of large quantities (particularly by sensitive or unaccustomed individuals) can lead to gas and bloating, and products which contain Inulin will sometimes include a warning to add it gradually to ones diet.; The inulin test is a procedure by which the filtering capacity of the glomeruli (the main filtering structures of the kidney) is determined by measuring the rate at which inulin, the test substance, is cleared from blood plasma. Inulin is one of the more suitable and accurate substance to measure because it is a small, inert polysaccharide molecule that readily passes through the glomeruli. The inulin clearance test is performed by injecting inulin, waiting for it to be distributed, and then measuring plasma and urine inulin concentrations by various assays. As nutraceutical agents inulins may have antitumor, antimicrobial, hypolipidemic and hypoglycemic actions. They may also help to improve mineral absorption and balance and may have antiosteoporotic activity.; There is a single report of what is claimed to be an allergic reaction to inulin in the literature, but dietary inulin has small amounts of bacteria and fungal spores and this case is most likely to represent a reaction to one of these contaminants:[citation needed] every day billions of people eat inulin-containing foods, e.g. onions, without any suggestion of allergy. Inulin is found in many foods, some of which are asparagus, endive, giant butterbur, and dandelion. |
|---|
| Contaminant Sources | - FooDB Chemicals
- HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
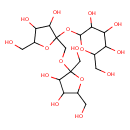
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C18H32O16 |
|---|
| Average Molecular Mass | 504.438 g/mol |
|---|
| Monoisotopic Mass | 504.169 g/mol |
|---|
| CAS Registry Number | 9005-80-5 |
|---|
| IUPAC Name | 2-{[2-({[3,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy}methyl)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]oxy}-6-(hydroxymethyl)oxane-3,4,5-triol |
|---|
| Traditional Name | 2-{[2-({[3,4-dihydroxy-2,5-bis(hydroxymethyl)oxolan-2-yl]oxy}methyl)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]oxy}-6-(hydroxymethyl)oxane-3,4,5-triol |
|---|
| SMILES | OCC1OC(CO)(OCC2(OC3OC(CO)C(O)C(O)C3O)OC(CO)C(O)C2O)C(O)C1O |
|---|
| InChI Identifier | InChI=1S/C18H32O16/c19-1-6-9(23)12(26)13(27)16(31-6)34-18(15(29)11(25)8(3-21)33-18)5-30-17(4-22)14(28)10(24)7(2-20)32-17/h6-16,19-29H,1-5H2 |
|---|
| InChI Key | VAWYEUIPHLMNNF-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oligosaccharides. These are carbohydrates made up of 3 to 10 monosaccharide units linked to each other through glycosidic bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Oligosaccharides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Oligosaccharide
- C-glycosyl compound
- Glycosyl compound
- O-glycosyl compound
- Ketal
- Oxane
- Tetrahydrofuran
- Secondary alcohol
- Acetal
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Alcohol
- Primary alcohol
- Hydrocarbon derivative
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01rx-0709000000-a0e999cfcd0c4ba2d6a8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03gi-0904000000-6e7de03e516009ded384 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-3900000000-4c950f3aa022d977816d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0200-1935100000-496b7cee843747524726 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01t9-0900000000-79c126044ff941a61712 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004m-4900000000-ec59df92e0092388cd8a | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | FDB001141 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Inulin |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 5045177 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|