| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:21:44 UTC |
|---|
| Update Date | 2016-11-09 01:17:26 UTC |
|---|
| Accession Number | CHEM022150 |
|---|
| Identification |
|---|
| Common Name | Dexmedetomidine |
|---|
| Class | Small Molecule |
|---|
| Description | An agonist of receptors, adrenergic alpha-2 that is used in veterinary medicine for its analgesic and sedative properties. It is the racemate of dexmedetomidine. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
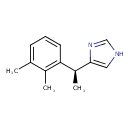
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+)-4-((S)-alpha,2,3-Trimethylbenzyl)imidazole | ChEBI | | Dexmedetomidina | ChEBI | | Dexmedetomidinum | ChEBI | | MPV 1440 | ChEBI | | Dexdor | Kegg | | Precedex | Kegg | | (+)-4-((S)-a,2,3-Trimethylbenzyl)imidazole | Generator | | (+)-4-((S)-Α,2,3-trimethylbenzyl)imidazole | Generator | | Medetomidine | HMDB | | Hospira brand OF dexmedetomidine hydrochloride | HMDB | | Hydrochloride, dexmedetomidine | HMDB | | Dexmedetomidine hydrochloride | HMDB |
|
|---|
| Chemical Formula | C13H16N2 |
|---|
| Average Molecular Mass | 200.280 g/mol |
|---|
| Monoisotopic Mass | 200.131 g/mol |
|---|
| CAS Registry Number | 113775-47-6 |
|---|
| IUPAC Name | 4-[(1S)-1-(2,3-dimethylphenyl)ethyl]-1H-imidazole |
|---|
| Traditional Name | dexmedetomidine hcl |
|---|
| SMILES | C[C@H](C1=CNC=N1)C1=C(C)C(C)=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C13H16N2/c1-9-5-4-6-12(10(9)2)11(3)13-7-14-8-15-13/h4-8,11H,1-3H3,(H,14,15)/t11-/m0/s1 |
|---|
| InChI Key | CUHVIMMYOGQXCV-NSHDSACASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as o-xylenes. These are aromatic compounds that contain a o-xylene moiety, which is a monocyclic benzene carrying exactly two methyl groups at the 1- and 2-positions. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Xylenes |
|---|
| Direct Parent | o-Xylenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - O-xylene
- Heteroaromatic compound
- Imidazole
- Azole
- Azacycle
- Organoheterocyclic compound
- Organic nitrogen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organonitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001r-3900000000-1af3ca78fb22ea06ac9d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0290000000-a37775586d59da8a5c1d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uk9-1950000000-1f473c29f9a4392cf069 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-1900000000-05caba97ecbe584d9c19 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-b4978ed90cadc637e3fb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-1900000000-b7da7a8cb234743e8c21 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0awc-4900000000-2ac7ce450e11fc5753d7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-1290000000-7ce023520ec9d746eea7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-7910000000-4ae55943a38333ee5287 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gdl-9800000000-cd3153463e2e91d489be | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-1900000000-03c59d592e0c63c3b129 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05r0-4900000000-63211e5f1011b3b9b21b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0690-4900000000-aa17f9adf1fe8fc096d3 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00633 |
|---|
| HMDB ID | HMDB0014771 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Dexmedetomidine |
|---|
| Chemspider ID | 4470605 |
|---|
| ChEBI ID | 4466 |
|---|
| PubChem Compound ID | 5311068 |
|---|
| Kegg Compound ID | C07450 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|