| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:21:07 UTC |
|---|
| Update Date | 2016-11-09 01:17:26 UTC |
|---|
| Accession Number | CHEM022132 |
|---|
| Identification |
|---|
| Common Name | Gadoversetamide |
|---|
| Class | Small Molecule |
|---|
| Description | Gadoversetamide, marketed under the trade name OptiMARK, is a gadolinium compound used as a contrast agent in magnetic resonance imaging (MRI), particularly imaging of the brain, spine and liver. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
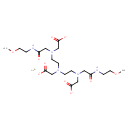
| Chemical Structure | |
|---|
| Synonyms | |
|---|
| Chemical Formula | C20H34GdN5O10 |
|---|
| Average Molecular Mass | 661.760 g/mol |
|---|
| Monoisotopic Mass | 662.155 g/mol |
|---|
| CAS Registry Number | 131069-91-5 |
|---|
| IUPAC Name | gadolinium(3+) ion 2-[bis({2-[(carboxylatomethyl)({[(2-methoxyethyl)carbamoyl]methyl})amino]ethyl})amino]acetate |
|---|
| Traditional Name | gadolinium(3+) ion 2-[bis({2-[(carboxylatomethyl)({[(2-methoxyethyl)carbamoyl]methyl})amino]ethyl})amino]acetate |
|---|
| SMILES | [Gd+3].COCCNC(=O)CN(CCN(CCN(CC([O-])=O)CC(=O)NCCOC)CC([O-])=O)CC([O-])=O |
|---|
| InChI Identifier | InChI=1S/C20H37N5O10.Gd/c1-34-9-3-21-16(26)11-24(14-19(30)31)7-5-23(13-18(28)29)6-8-25(15-20(32)33)12-17(27)22-4-10-35-2;/h3-15H2,1-2H3,(H,21,26)(H,22,27)(H,28,29)(H,30,31)(H,32,33);/q;+3/p-3 |
|---|
| InChI Key | HBEAOBRDTOXWRZ-UHFFFAOYSA-K |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as alpha amino acid amides. These are amide derivatives of alpha amino acids. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Alpha amino acid amides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-amino acid amide
- Alpha-amino acid
- Tricarboxylic acid or derivatives
- Carboxamide group
- Carboxylic acid salt
- Secondary carboxylic acid amide
- Tertiary amine
- Tertiary aliphatic amine
- Amino acid
- Ether
- Dialkyl ether
- Carboxylic acid
- Carbonyl group
- Amine
- Organic oxygen compound
- Organooxygen compound
- Organonitrogen compound
- Organic nitrogen compound
- Organopnictogen compound
- Organic zwitterion
- Organic salt
- Hydrocarbon derivative
- Organic oxide
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-03di-2031901000-7138d3d3f1b5d5d82c31 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-1006902000-595b71fa606d448127bc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-02di-7439300000-8e81ec2ad697367661db | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-1119000000-96bf72003f50228ff7b3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0000904000-8dd04c17d8b56f4c5205 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03k9-2010901000-8f060f6d50f1454e814b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00dl-9112400000-efda83802de0d6bca04b | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00538 |
|---|
| HMDB ID | HMDB0014678 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Gadoversetamide |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 31644 |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|