| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:20:51 UTC |
|---|
| Update Date | 2016-11-09 01:17:25 UTC |
|---|
| Accession Number | CHEM022122 |
|---|
| Identification |
|---|
| Common Name | Tridihexethyl |
|---|
| Class | Small Molecule |
|---|
| Description | Tridihexethyl is only found in individuals that have used or taken this drug. It is a synthetic anticholinergic agent which has been shown in experimental and clinical studies to have a pronounced antispasmodic and antisecretory effect on the gastrointestinal tract. Tridihexethyl is an antimuscarinic, anticholinergic drug.Tridihexethyl binds the muscarinic acetylcholine receptor. It may block all three types of muscarinic receptors including M-1 receptors in the CNS and ganglia, M-2 receptors in the heart (vagus) and M-3 receptors at the parasympathetic NEJ system. The muscarinic acetylcholine receptors mediate various cellular responses, including inhibition of adenylate cyclase, breakdown of phosphoinositides and modulation of potassium channels through the action of G proteins. Tridihexethyl inhibits vagally mediated reflexes by antagonizing the action of acetylcholine. This in turn reduces the secretion of gastric acids in the stomach. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
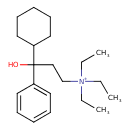
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Propethonum | ChEBI | | Tridihexethyl iodide | HMDB | | Tridihexylethyl chloride | HMDB | | (3-Cyclohexyl-3-hydroxy-3-phenylpropyl)triethylammonium iodide | HMDB | | Tridihexethyl decylsulfate | HMDB | | Pathilon | HMDB | | Chloride OF tridihexethyl | HMDB |
|
|---|
| Chemical Formula | C21H36NO |
|---|
| Average Molecular Mass | 318.517 g/mol |
|---|
| Monoisotopic Mass | 318.280 g/mol |
|---|
| CAS Registry Number | 60-49-1 |
|---|
| IUPAC Name | (3-cyclohexyl-3-hydroxy-3-phenylpropyl)triethylazanium |
|---|
| Traditional Name | tridihexethyl |
|---|
| SMILES | CC[N+](CC)(CC)CCC(O)(C1CCCCC1)C1=CC=CC=C1 |
|---|
| InChI Identifier | InChI=1S/C21H36NO/c1-4-22(5-2,6-3)18-17-21(23,19-13-9-7-10-14-19)20-15-11-8-12-16-20/h7,9-10,13-14,20,23H,4-6,8,11-12,15-18H2,1-3H3/q+1 |
|---|
| InChI Key | NPRHVSBSZMAEIN-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as aralkylamines. These are alkylamines in which the alkyl group is substituted at one carbon atom by an aromatic hydrocarbyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic nitrogen compounds |
|---|
| Class | Organonitrogen compounds |
|---|
| Sub Class | Amines |
|---|
| Direct Parent | Aralkylamines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aralkylamine
- Monocyclic benzene moiety
- Benzenoid
- 1,3-aminoalcohol
- Tetraalkylammonium salt
- Quaternary ammonium salt
- Tertiary alcohol
- Organic oxygen compound
- Alcohol
- Aromatic alcohol
- Organic salt
- Hydrocarbon derivative
- Organooxygen compound
- Organopnictogen compound
- Organic cation
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0a59-8971000000-2ecee937b21e78ee8b21 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0c29-4296000000-66242f6ffe50c7e1393d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0009000000-8740c81da0de6d35bbc6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0wmi-3933000000-b934d2d3b2f3a2b1357f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f79-9410000000-390e4eb521f92aeb1b63 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-1639000000-c616b0c7af47dd96b29f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udr-8930000000-dc7166aed4c606073c7c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0uki-8900000000-fe7fb13296d83ecaeb54 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00505 |
|---|
| HMDB ID | HMDB0014648 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Tridihexethyl |
|---|
| Chemspider ID | 19124 |
|---|
| ChEBI ID | 9701 |
|---|
| PubChem Compound ID | 20299 |
|---|
| Kegg Compound ID | C07861 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|