Colestipol (CHEM022101)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 1.0 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2016-05-25 18:20:01 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2016-11-09 01:17:25 UTC | ||||||||||||||||||||||||||||||||||||||||||||||||
| Accession Number | CHEM022101 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||
| Common Name | Colestipol | ||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Small Molecule | ||||||||||||||||||||||||||||||||||||||||||||||||
| Description | Bile acid sequestrants like colestipol have been in use since the 1970s. And even though such an agent may very well be useful in reducing elevated cholesterol levels and decreasing the risk for atherosclerotic vascular disease due to hypercholesterolemia, colestipol is still generally only employed as an adjunct therapy and the relatively physical nature of its pharmacological activity sometimes limits its usefulness. In particular, as colestipol's general mechanism of action ultimately results in the decreased absorption and enhanced secretion of bile acids and lipids in the feces, patients who take complicated medication regimens, experience constipation or biliary obstruction, etc. may not be good candidates for using the agent owing to its physical effects on the gut. Alternatively, colestipol predominantly elicits its activities within the gut environment because it undergoes little absorption and metabolism. The resultant lack of systemic exposure consequently means the medication generally demonstrates very few adverse effects inside the body. | ||||||||||||||||||||||||||||||||||||||||||||||||
| Contaminant Sources |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Contaminant Type | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
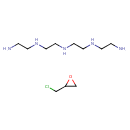
| Chemical Structure | |||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula | C11H28ClN5O | ||||||||||||||||||||||||||||||||||||||||||||||||
| Average Molecular Mass | 281.826 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| Monoisotopic Mass | 281.198 g/mol | ||||||||||||||||||||||||||||||||||||||||||||||||
| CAS Registry Number | 50925-79-6 | ||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name | (2-aminoethyl)[2-({2-[(2-aminoethyl)amino]ethyl}amino)ethyl]amine; 2-(chloromethyl)oxirane | ||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional Name | epichlorohydrin; tetraethylenepentamine | ||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES | ClCC1CO1.NCCNCCNCCNCCN | ||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Identifier | InChI=1S/C8H23N5.C3H5ClO/c9-1-3-11-5-7-13-8-6-12-4-2-10;4-1-3-2-5-3/h11-13H,1-10H2;3H,1-2H2 | ||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key | GMRWGQCZJGVHKL-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as epoxides. Epoxides are compounds containing a cyclic ether with three ring atoms(one oxygen and two carbon atoms). | ||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organoheterocyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Epoxides | ||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Epoxides | ||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||
| Status | Detected and Not Quantified | ||||||||||||||||||||||||||||||||||||||||||||||||
| Origin | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Biofluid Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Tissue Locations | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Pathways | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Applications | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Roles | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Roles | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||
| State | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra |
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Profile | |||||||||||||||||||||||||||||||||||||||||||||||||
| Route of Exposure | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Mechanism of Toxicity | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Metabolism | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Toxicity Values | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Lethal Dose | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Carcinogenicity (IARC Classification) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Uses/Sources | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Minimum Risk Level | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Health Effects | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Symptoms | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Treatment | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||
| External Links | |||||||||||||||||||||||||||||||||||||||||||||||||
| DrugBank ID | DB00375 | ||||||||||||||||||||||||||||||||||||||||||||||||
| HMDB ID | HMDB0014519 | ||||||||||||||||||||||||||||||||||||||||||||||||
| FooDB ID | FDB010378 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Phenol Explorer ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| KNApSAcK ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| BiGG ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| BioCyc ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| METLIN ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| PDB ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| Wikipedia Link | Colestipol | ||||||||||||||||||||||||||||||||||||||||||||||||
| Chemspider ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| ChEBI ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| PubChem Compound ID | 62816 | ||||||||||||||||||||||||||||||||||||||||||||||||
| Kegg Compound ID | C06925 | ||||||||||||||||||||||||||||||||||||||||||||||||
| YMDB ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| ECMDB ID | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| MSDS | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||
| General References | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||