| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:19:25 UTC |
|---|
| Update Date | 2016-11-09 01:17:25 UTC |
|---|
| Accession Number | CHEM022080 |
|---|
| Identification |
|---|
| Common Name | Cefmetazole |
|---|
| Class | Small Molecule |
|---|
| Description | A semisynthetic cephamycin antibiotic with a broad spectrum of activity against both gram-positive and gram-negative microorganisms. It has a high rate of efficacy in many types of infection and to date no severe side effects have been noted. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
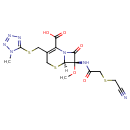
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (6R,7S)-7-({[(cyanomethyl)sulfanyl]acetyl}amino)-7-methoxy-3-{[(1-methyl-1H-tetrazol-5-yl)sulfanyl]methyl}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid | ChEBI | | Cefmetazolo | ChEBI | | Cefmetazolum | ChEBI | | CMZ | Kegg | | (6R,7S)-7-({[(cyanomethyl)sulfanyl]acetyl}amino)-7-methoxy-3-{[(1-methyl-1H-tetrazol-5-yl)sulfanyl]methyl}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate | Generator | | (6R,7S)-7-({[(cyanomethyl)sulphanyl]acetyl}amino)-7-methoxy-3-{[(1-methyl-1H-tetrazol-5-yl)sulphanyl]methyl}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate | Generator | | (6R,7S)-7-({[(cyanomethyl)sulphanyl]acetyl}amino)-7-methoxy-3-{[(1-methyl-1H-tetrazol-5-yl)sulphanyl]methyl}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid | Generator | | Monosodium salt, cefmetazole | HMDB | | Zefazone | HMDB | | Cefmetazon | HMDB | | Pharmacia brand OF cefmetazole sodium | HMDB | | Salt, cefmetazole monosodium | HMDB | | Cefmetazole monosodium salt | HMDB | | Cefmetazole sodium | HMDB | | Sodium, cefmetazole | HMDB |
|
|---|
| Chemical Formula | C15H17N7O5S3 |
|---|
| Average Molecular Mass | 471.534 g/mol |
|---|
| Monoisotopic Mass | 471.045 g/mol |
|---|
| CAS Registry Number | 56796-20-4 |
|---|
| IUPAC Name | (6R,7S)-7-{2-[(cyanomethyl)sulfanyl]acetamido}-7-methoxy-3-{[(1-methyl-1H-1,2,3,4-tetrazol-5-yl)sulfanyl]methyl}-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylic acid |
|---|
| Traditional Name | cefmetazole |
|---|
| SMILES | [H][C@]12SCC(CSC3=NN=NN3C)=C(N1C(=O)[C@]2(NC(=O)CSCC#N)OC)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C15H17N7O5S3/c1-21-14(18-19-20-21)30-6-8-5-29-13-15(27-2,17-9(23)7-28-4-3-16)12(26)22(13)10(8)11(24)25/h13H,4-7H2,1-2H3,(H,17,23)(H,24,25)/t13-,15+/m1/s1 |
|---|
| InChI Key | SNBUBQHDYVFSQF-HIFRSBDPSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as n-acyl-alpha amino acids and derivatives. N-acyl-alpha amino acids and derivatives are compounds containing an alpha amino acid (or a derivative thereof) which bears an acyl group at its terminal nitrogen atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | N-acyl-alpha amino acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - N-acyl-alpha amino acid or derivatives
- Cephem
- Aryl thioether
- Alkylarylthioether
- Meta-thiazine
- Azole
- Beta-lactam
- Heteroaromatic compound
- Tertiary carboxylic acid amide
- Tetrazole
- Azetidine
- Carboxamide group
- Lactam
- Carboximidic acid
- Carboximidic acid derivative
- Carboxylic acid
- Azacycle
- Organoheterocyclic compound
- Monocarboxylic acid or derivatives
- Dialkylthioether
- Carbonitrile
- Nitrile
- Organic 1,3-dipolar compound
- Sulfenyl compound
- Hemithioaminal
- Thioether
- Propargyl-type 1,3-dipolar organic compound
- Hydrocarbon derivative
- Organonitrogen compound
- Organooxygen compound
- Organosulfur compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Carbonyl group
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9252300000-3b897b33c8f75fb7d195 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-05bf-9212320000-890b51c43c3c2ab21ca4 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-1043900000-6c51b4b1826d8fd2c6f0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01x3-7940000000-c539600c8e5309da2623 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03kl-5393000000-620b60fde9e0798480d5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00bc-2434900000-d70717dd74e62de2b0f5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-9341200000-9e56fcac14c64926ea08 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-044i-2191000000-2f3da3edb938f355e383 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0300900000-77e091905e6148c9a4f8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00xr-4850900000-81fd58ff3fbb9a095c81 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-9400000000-7442047ce9e5c1ddf3f3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-05fr-0008900000-09f33a45a1206aa0b044 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-4104900000-28beec94cf5d21f8447a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9102100000-234a13d09fa139832d83 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00274 |
|---|
| HMDB ID | HMDB0014419 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Cefmetazole |
|---|
| Chemspider ID | 38311 |
|---|
| ChEBI ID | 3489 |
|---|
| PubChem Compound ID | 42008 |
|---|
| Kegg Compound ID | C08103 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|