| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:18:48 UTC |
|---|
| Update Date | 2016-11-09 01:17:25 UTC |
|---|
| Accession Number | CHEM022060 |
|---|
| Identification |
|---|
| Common Name | Tetrahydrofolic acid |
|---|
| Class | Small Molecule |
|---|
| Description | Tetrahydrofolic acid, also known as tetrahydrofolic acid, belongs to the class of organic compounds known as tetrahydrofolic acids. These are heterocyclic compounds based on the 5,6,7,8-tetrahydropteroic acid skeleton conjugated with at least one L-glutamic acid unit. Tetrahydrofolic acid is possibly soluble (in water) and a strong basic compound (based on its pKa). Tetrahydrofolic acid exists in all eukaryotes, ranging from yeast to humans. Tetrahydrofolic acid participates in a number of enzymatic reactions, within cattle. In particular, Tetrahydrofolic acid can be converted into dihydrofolic acid through its interaction with the enzyme dihydrofolate reductase. In addition, Tetrahydrofolic acid and formic acid can be converted into 10-formyltetrahydrofolic acid; which is mediated by the enzyme C-1-tetrahydrofolic acid synthase, cytoplasmic. In cattle, tetrahydrofolic acid is involved in the metabolic pathway called the folate metabolism pathway. |
|---|
| Contaminant Sources | - FooDB Chemicals
- HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
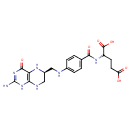
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Tetrahydrofolate | Generator | | (6S)-Tetrahydrofolate | HMDB | | (6S)-Tetrahydrofolic acid | HMDB | | 5,6,7,8-Tetrahydrofolate | HMDB | | 5,6,7,8-Tetrahydrofolic acid | HMDB | | Tetra-H-folate | HMDB | | Tetrahydrafolate | HMDB | | Tetrahydropteroyl mono-L-glutamate | HMDB | | Tetrahydropteroylglutamate | HMDB |
|
|---|
| Chemical Formula | C19H23N7O6 |
|---|
| Average Molecular Mass | 445.429 g/mol |
|---|
| Monoisotopic Mass | 445.171 g/mol |
|---|
| CAS Registry Number | 135-16-0 |
|---|
| IUPAC Name | 2-{[4-({[(6S)-4-hydroxy-2-imino-1,2,5,6,7,8-hexahydropteridin-6-yl]methyl}amino)phenyl]formamido}pentanedioic acid |
|---|
| Traditional Name | 2-{[4-({[(6S)-4-hydroxy-2-imino-5,6,7,8-tetrahydro-1H-pteridin-6-yl]methyl}amino)phenyl]formamido}pentanedioic acid |
|---|
| SMILES | NC1=NC(=O)C2=C(NC[C@H](CNC3=CC=C(C=C3)C(=O)NC(CCC(O)=O)C(O)=O)N2)N1 |
|---|
| InChI Identifier | InChI=1S/C19H23N7O6/c20-19-25-15-14(17(30)26-19)23-11(8-22-15)7-21-10-3-1-9(2-4-10)16(29)24-12(18(31)32)5-6-13(27)28/h1-4,11-12,21,23H,5-8H2,(H,24,29)(H,27,28)(H,31,32)(H4,20,22,25,26,30)/t11-,12?/m0/s1 |
|---|
| InChI Key | MSTNYGQPCMXVAQ-PXYINDEMSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as xanthines. These are purine derivatives with a ketone group conjugated at carbons 2 and 6 of the purine moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Imidazopyrimidines |
|---|
| Sub Class | Purines and purine derivatives |
|---|
| Direct Parent | Xanthines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Xanthine
- Purinone
- 6-oxopurine
- Alkaloid or derivatives
- Pyrimidone
- Pyrimidine
- N-substituted imidazole
- Heteroaromatic compound
- Vinylogous amide
- Imidazole
- Azole
- Urea
- Lactam
- Azacycle
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0uka-4676900000-780e78301e85c240ab73 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-006x-1311190000-af98cc8be4da08f9b782 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0002-7796610000-0937514a7f23431b726e | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-0nor-3269210000-20105ed6a7a6e24e2441 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-00kr-1935200000-a9449c19c1ae3b0d70da | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0032-0431900000-ff1f3948e47561161293 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0952300000-ed9d84bc39d490f6210e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-001i-0920000000-0d0efcb6c323340d20b9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0001900000-abb4cec0eb79b0bd2de1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0v4l-2356900000-2fd17d2f587bbd5e882c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9441000000-8c630c7a3633467d6bb3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0080900000-73480c24ce118e5e02f3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0491100000-0b5df4d0f168f8f15275 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0089-0962000000-9a988cf519f340f5d7cf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-005c-0002900000-9587282a39f915386f95 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0zi3-2807900000-28355749389d1e225f7b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-3911000000-e7b1ccb0c9f2d7c8269a | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 2D NMR | [1H,1H] 2D NMR Spectrum | Not Available | Spectrum | | 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0001846 |
|---|
| FooDB ID | FDB022705 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | 33856 |
|---|
| BioCyc ID | THF |
|---|
| METLIN ID | 714 |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Tetrahydrofolic acid |
|---|
| Chemspider ID | 82572 |
|---|
| ChEBI ID | 20506 |
|---|
| PubChem Compound ID | 91443 |
|---|
| Kegg Compound ID | C00101 |
|---|
| YMDB ID | YMDB00139 |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Garbis SD, Melse-Boonstra A, West CE, van Breemen RB: Determination of folates in human plasma using hydrophilic interaction chromatography-tandem mass spectrometry. Anal Chem. 2001 Nov 15;73(22):5358-64. | | 2. Authors unspecified: Pemetrexed: new drug. Pleural mesothelioma: a first encouraging trial. Prescrire Int. 2005 Dec;14(80):212-4. | | 3. Asrar FM, O'Connor DL: Bacterially synthesized folate and supplemental folic acid are absorbed across the large intestine of piglets. J Nutr Biochem. 2005 Oct;16(10):587-93. | | 4. Ahmed F, Khan MR, Akhtaruzzaman M, Karim R, Marks GC, Banu CP, Nahar B, Williams G: Efficacy of twice-weekly multiple micronutrient supplementation for improving the hemoglobin and micronutrient status of anemic adolescent schoolgirls in Bangladesh. Am J Clin Nutr. 2005 Oct;82(4):829-35. | | 5. Pieniazek D, Kubalska J, Pronicka E, Stecko E: Disturbances in histidine metabolism in children with speech abnormalities. Acta Anthropogenet. 1985;9(1-3):117-21. | | 6. Ozer B, Serin E, Gumurdulu Y, Kayaselcuk F, Anarat R, Gur G, Kul K, Guclu M, Boyacioglu S: Helicobacter pylori eradication lowers serum homocysteine level in patients without gastric atrophy. World J Gastroenterol. 2005 May 14;11(18):2764-7. | | 7. Greenwald P, Milner JA, Anderson DE, McDonald SS: Micronutrients in cancer chemoprevention. Cancer Metastasis Rev. 2002;21(3-4):217-30. | | 8. Siega-Riz AM, Savitz DA, Zeisel SH, Thorp JM, Herring A: Second trimester folate status and preterm birth. Am J Obstet Gynecol. 2004 Dec;191(6):1851-7. | | 9. Taber LD, O'Brien P, Bowsher RR, Sportsman JR: Competitive particle concentration fluorescence immunoassay for measuring 5,10-dideaza-5,6,7,8-tetrahydrofolic acid (lometrexol) in serum. Clin Chem. 1991 Feb;37(2):254-60. | | 10. Mattson MP: Gene-diet interactions in brain aging and neurodegenerative disorders. Ann Intern Med. 2003 Sep 2;139(5 Pt 2):441-4. | | 11. Dietrich M, Brown CJ, Block G: The effect of folate fortification of cereal-grain products on blood folate status, dietary folate intake, and dietary folate sources among adult non-supplement users in the United States. J Am Coll Nutr. 2005 Aug;24(4):266-74. | | 12. Kamen BA, Smith AK: A review of folate receptor alpha cycling and 5-methyltetrahydrofolate accumulation with an emphasis on cell models in vitro. Adv Drug Deliv Rev. 2004 Apr 29;56(8):1085-97. | | 13. Baggott JE, Johanning GL, Branham KE, Prince CW, Morgan SL, Eto I, Vaughn WH: Cofactor role for 10-formyldihydrofolic acid. Biochem J. 1995 Jun 15;308 ( Pt 3):1031-6. | | 14. Makino Y, Nagano M, Tamura K, Kawarabayashi T: Pregnancy complicated with pure red cell aplasia: a case report. J Perinat Med. 2003;31(6):530-4. | | 15. Pljesa S: [Possible complications of erythropoietin therapy in patients with chronic renal failure]. Med Pregl. 2004 May-Jun;57(5-6):254-7. | | 16. Pufulete M, Al-Ghnaniem R, Khushal A, Appleby P, Harris N, Gout S, Emery PW, Sanders TA: Effect of folic acid supplementation on genomic DNA methylation in patients with colorectal adenoma. Gut. 2005 May;54(5):648-53. | | 17. Wang S, Low PS: Folate-mediated targeting of antineoplastic drugs, imaging agents, and nucleic acids to cancer cells. J Control Release. 1998 Apr 30;53(1-3):39-48. | | 18. Hankey GJ, Eikelboom JW, Loh K, Tang M, Pizzi J, Thom J, Yi Q: Sustained homocysteine-lowering effect over time of folic acid-based multivitamin therapy in stroke patients despite increasing folate status in the population. Cerebrovasc Dis. 2005;19(2):110-6. Epub 2004 Dec 17. | | 19. Ramaekers VT, Rothenberg SP, Sequeira JM, Opladen T, Blau N, Quadros EV, Selhub J: Autoantibodies to folate receptors in the cerebral folate deficiency syndrome. N Engl J Med. 2005 May 12;352(19):1985-91. | | 20. Verwei M, Arkbage K, Mocking H, Havenaar R, Groten J: The binding of folic acid and 5-methyltetrahydrofolate to folate-binding proteins during gastric passage differs in a dynamic in vitro gastrointestinal model. J Nutr. 2004 Jan;134(1):31-7. | | 21. Mattson MP: Will caloric restriction and folate protect against AD and PD? Neurology. 2003 Feb 25;60(4):690-5. | | 22. Omura Y: Excessive use of Steroid Hormone & beneficial effects of True St. 36 acupuncture on malignant brain tumors--part I; how to estimate non-invasively presence of excess dose of Steroid Hormone in patients, baseball players & other professional athletes from its toxic effects on heart & pancreas, as well as persistent or recurrent infection--part II. Acupunct Electrother Res. 2005;30(1-2):57-102. | | 23. Paulionis L, Kane SL, Meckling KA: Vitamin status and cognitive function in a long-term care population. BMC Geriatr. 2005 Dec 13;5:16. | | 24. Zhu WY, Alliegro MA, Melera PW: The rate of folate receptor alpha (FR alpha) synthesis in folate depleted CHL cells is regulated by a translational mechanism sensitive to media folate levels, while stable overexpression of its mRNA is mediated by gene amplification and an increase in transcript half-life. J Cell Biochem. 2001 Mar 26;81(2):205-19. | | 25. Tchantchou F: Homocysteine metabolism and various consequences of folate deficiency. J Alzheimers Dis. 2006 Aug;9(4):421-7. | | 26. Lu S, Chen GL, Ren C, Kwabi-Addo B, Epner DE: Methionine restriction selectively targets thymidylate synthase in prostate cancer cells. Biochem Pharmacol. 2003 Sep 1;66(5):791-800. | | 27. Durga J, van Boxtel MP, Schouten EG, Bots ML, Kok FJ, Verhoef P: Folate and the methylenetetrahydrofolate reductase 677C-->T mutation correlate with cognitive performance. Neurobiol Aging. 2006 Feb;27(2):334-43. Epub 2005 Feb 24. | | 28. Stuerenburg HJ, Ganzer S, Arlt S, Muller-Thomsen T: The influence of smoking on plasma folate and lipoproteins in Alzheimer disease, mild cognitive impairment and depression. Neuro Endocrinol Lett. 2005 Jun;26(3):261-3. | | 29. Smith DE, Kok RM, Teerlink T, Jakobs C, Smulders YM: Quantitative determination of erythrocyte folate vitamer distribution by liquid chromatography-tandem mass spectrometry. Clin Chem Lab Med. 2006;44(4):450-9. | | 30. Wolters M, Strohle A, Hahn A: [Age-associated changes in the metabolism of vitamin B(12) and folic acid: prevalence, aetiopathogenesis and pathophysiological consequences]. Z Gerontol Geriatr. 2004 Apr;37(2):109-35. | | 31. Sahr T, Ravanel S, Rebeille F: Tetrahydrofolate biosynthesis and distribution in higher plants. Biochem Soc Trans. 2005 Aug;33(Pt 4):758-62. |
|
|---|