| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:18:46 UTC |
|---|
| Update Date | 2016-11-09 01:17:25 UTC |
|---|
| Accession Number | CHEM022059 |
|---|
| Identification |
|---|
| Common Name | Cetrorelix |
|---|
| Class | Small Molecule |
|---|
| Description | A synthetic ten-membered oligopeptide comprising N-acetyl-3-(naphthalen-2-yl)-D-alanyl, 4-chloro-D-phenylalanyl, 3-(pyridin-3-yl)-D-alanyl, L-seryl, L-tyrosyl, N(5)-carbamoyl-D-ornithyl, L-leucyl, L-arginyl, L-prolyl, and D-alaninamide residues coupled in sequence. A gonadotrophin-releasing hormone (GnRH) antagonist, it is used for treatment of infertility and of hormone-sensitive cancers of the prostate and breast. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
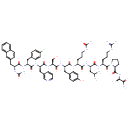
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Cetrorelixum | ChEBI | | Cetrotide | Kegg | | SB 75 | HMDB | | SB-75 | HMDB | | N-Acetyl-1-(3-(2-naphthyl)alanine)-2-(4-chlorophenylalanine)-3-(3-(3-pyridyl)alanine)-6-citrulline-10-alanine-LHRH | HMDB | | Cetrorelix acetate | HMDB | | Cetrorelix pamoate | HMDB |
|
|---|
| Chemical Formula | C70H92ClN17O14 |
|---|
| Average Molecular Mass | 1431.038 g/mol |
|---|
| Monoisotopic Mass | 1429.670 g/mol |
|---|
| CAS Registry Number | 120287-85-6 |
|---|
| IUPAC Name | (2S)-N-[(2S)-5-carbamimidamido-1-[(2S)-2-{[(1R)-1-carbamoylethyl]carbamoyl}pyrrolidin-1-yl]-1-oxopentan-2-yl]-2-[(2R)-5-(carbamoylamino)-2-[(2S)-2-[(2S)-2-[(2R)-2-[(2R)-3-(4-chlorophenyl)-2-[(2R)-2-acetamido-3-(naphthalen-2-yl)propanamido]propanamido]-3-(pyridin-3-yl)propanamido]-3-hydroxypropanamido]-3-(4-hydroxyphenyl)propanamido]pentanamido]-4-methylpentanamide |
|---|
| Traditional Name | cetrorelix |
|---|
| SMILES | CC(C)C[C@H](NC(=O)[C@@H](CCCNC(N)=O)NC(=O)[C@H](CC1=CC=C(O)C=C1)NC(=O)[C@H](CO)NC(=O)[C@@H](CC1=CN=CC=C1)NC(=O)[C@@H](CC1=CC=C(Cl)C=C1)NC(=O)[C@@H](CC1=CC2=CC=CC=C2C=C1)NC(C)=O)C(=O)N[C@@H](CCCNC(N)=N)C(=O)N1CCC[C@H]1C(=O)N[C@H](C)C(N)=O |
|---|
| InChI Identifier | InChI=1S/C70H92ClN17O14/c1-39(2)31-52(61(94)82-51(15-9-28-77-69(73)74)68(101)88-30-10-16-58(88)67(100)79-40(3)59(72)92)83-60(93)50(14-8-29-78-70(75)102)81-63(96)54(34-43-20-25-49(91)26-21-43)86-66(99)57(38-89)87-65(98)56(36-45-11-7-27-76-37-45)85-64(97)55(33-42-18-23-48(71)24-19-42)84-62(95)53(80-41(4)90)35-44-17-22-46-12-5-6-13-47(46)32-44/h5-7,11-13,17-27,32,37,39-40,50-58,89,91H,8-10,14-16,28-31,33-36,38H2,1-4H3,(H2,72,92)(H,79,100)(H,80,90)(H,81,96)(H,82,94)(H,83,93)(H,84,95)(H,85,97)(H,86,99)(H,87,98)(H4,73,74,77)(H3,75,78,102)/t40-,50-,51+,52+,53-,54+,55-,56-,57+,58+/m1/s1 |
|---|
| InChI Key | SBNPWPIBESPSIF-MHWMIDJBSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as polypeptides. These are peptides containing ten or more amino acid residues. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic Polymers |
|---|
| Class | Polypeptides |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Polypeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Polypeptide
- Alpha peptide
- Tyrosine or derivatives
- Phenylalanine or derivatives
- Leucine or derivatives
- Proline or derivatives
- N-acyl-alpha amino acid or derivatives
- Alpha-amino acid amide
- Serine or derivatives
- Alanine or derivatives
- Naphthalene
- N-substituted-alpha-amino acid
- Alpha-amino acid or derivatives
- Amphetamine or derivatives
- N-acylpyrrolidine
- Pyrrolidine carboxylic acid or derivatives
- Pyrrolidine-2-carboxamide
- Chlorobenzene
- Halobenzene
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Benzenoid
- Aryl halide
- Fatty amide
- Aryl chloride
- Pyridine
- N-acyl-amine
- Monocyclic benzene moiety
- Fatty acyl
- Tertiary carboxylic acid amide
- Pyrrolidine
- Heteroaromatic compound
- Acetamide
- Carboxamide group
- Urea
- Guanidine
- Secondary carboxylic acid amide
- Primary carboxylic acid amide
- Carbonic acid derivative
- Azacycle
- Organoheterocyclic compound
- Carboxylic acid derivative
- Carboximidamide
- Organochloride
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Alcohol
- Carbonyl group
- Imine
- Organic oxide
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Organohalogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03du-5119702200-713925e521f82910ba30 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01p6-9247614220-9f57159f8b39e33cb6f4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03du-9244111100-c5289fceb123c9db71ea | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03y0-2009300000-fdcb67df57c3c50e2b4d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0cdl-5109300001-d9e1a7fe30de34be3f74 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9011010111-f8b524a667f58a9d9b4e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-2016915000-1a4cf2b7028e88b02ae0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0296-5109610101-a9f98cc0eb4083fc6986 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9633110201-bbc7753fab5fde409e50 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03l0-0207910010-10cc81c2d8dfc0daaafe | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01ow-9544543364-67bc4a9477b67e8e3186 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f6y-5692060120-1673f665533503b2e3d4 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB00050 |
|---|
| HMDB ID | HMDB0014261 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Cetrorelix |
|---|
| Chemspider ID | 10482082 |
|---|
| ChEBI ID | 59224 |
|---|
| PubChem Compound ID | 25074887 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|