| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:17:59 UTC |
|---|
| Update Date | 2016-11-09 01:17:24 UTC |
|---|
| Accession Number | CHEM022018 |
|---|
| Identification |
|---|
| Common Name | Donepezil metabolite M4 |
|---|
| Class | Small Molecule |
|---|
| Description | Donepezil metabolite M4 is a metabolite of Donepezil. Donepezil, marketed under the trade name Aricept by its developer Eisai and partner Pfizer, and also marketed under the brand name DONEP by Alkem Pentacare, is a centrally acting reversible acetylcholinesterase inhibitor. Its main therapeutic use is in the palliative treatment of Alzheimer's disease. Common side effects include gastrointestinal upset. It has an oral bioavailability of 100% and easily crosses the blood–brain barrier. Because it has a biological half-life of about 70 hours, it can be taken once a day. (Wikipedia) |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
- STOFF IDENT Compounds
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (2S,3R,4E)-2-Alkanamido-3-hydroxyoctadec-4-en-1-yl 3-O-(5-acetamido-3,5-dideoxy-D-glycero-alpha-D-galacto-non-2-ulopyranonosyl)-beta-D-galactopyranoside | HMDB | | (2S,3R,4E)-2-Alkanamido-3-hydroxyoctadec-4-en-1-yl 5-acetamido-3,5-dideoxy-D-glycero-alpha-D-galacto-non-2-ulopyranonosyl-(2Right3)-beta-D-galactopyranoside | HMDB | | (Acetyloxy)(tributyl)stannane (acd/name 4.0) | HMDB | | (Acetyloxy)tributyl-stannane | HMDB | | (Gal)1 (neu5ac)1 (cer)1 | HMDB | | Acetoxytributyl-stannane | HMDB | | Acetoxytributyl-tin | HMDB | | Acetoxytributylstannane | HMDB | | Acetoxytributyltin | HMDB | | alpha-Neu5ac-(2Right3)-beta-D-gal-(1leftright1')-cer | HMDB | | alpha-Neup5ac-(2Right3)-beta-D-galp-(1leftright1')-cer | HMDB | | Ganglioside GM4 | HMDB | | GM4 | HMDB | | N-Acetyl-alpha-neuraminosyl-(2Right3)-beta-D-galactosylceramides | HMDB | | N-Acetylneuraminyl-galactosylceramide | HMDB | | Neu5ac-alpha2->3gal-beta1->1'cer | HMDB | | Neu5ac-gal-beta1->1'cer | HMDB | | NeuAcalpha2-3galbeta-cer | HMDB | | TBTA | HMDB | | Tin, tributyl-, acetate | HMDB | | Tri-N-butyltin acetate | HMDB | | Tributylacetoxystannane | HMDB | | Tributylstannyl acetate | HMDB | | Tributyltin acetate | HMDB | | Tribuyltin acetate | HMDB |
|
|---|
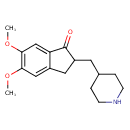
| Chemical Formula | C17H23NO3 |
|---|
| Average Molecular Mass | 289.369 g/mol |
|---|
| Monoisotopic Mass | 289.168 g/mol |
|---|
| CAS Registry Number | 56-36-0 |
|---|
| IUPAC Name | 5,6-dimethoxy-2-(piperidin-4-ylmethyl)-2,3-dihydro-1H-inden-1-one |
|---|
| Traditional Name | 5,6-dimethoxy-2-(piperidin-4-ylmethyl)-2,3-dihydroinden-1-one |
|---|
| SMILES | COC1=C(OC)C=C2C(=O)C(CC3CCNCC3)CC2=C1 |
|---|
| InChI Identifier | InChI=1S/C17H23NO3/c1-20-15-9-12-8-13(7-11-3-5-18-6-4-11)17(19)14(12)10-16(15)21-2/h9-11,13,18H,3-8H2,1-2H3 |
|---|
| InChI Key | PGBZORAISITZTF-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as indanones. Indanones are compounds containing an indane ring bearing a ketone group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Indanes |
|---|
| Sub Class | Indanones |
|---|
| Direct Parent | Indanones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Indanone
- Anisole
- Aryl ketone
- Aryl alkyl ketone
- Alkyl aryl ether
- Aralkylamine
- Piperidine
- Ketone
- Secondary aliphatic amine
- Ether
- Secondary amine
- Azacycle
- Organoheterocyclic compound
- Organonitrogen compound
- Organic oxide
- Organopnictogen compound
- Amine
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxygen compound
- Organic nitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-008a-7390000000-30b373dbaf089a113774 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0090000000-da28a41bd1b829d71a8e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0bu3-4970000000-801d3c0b32c93fd11165 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a59-9510000000-6d1d9c2e6a3d68e3dff0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-e07661d76997ea63c579 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-1190000000-7f3cb62ab8e739b13f28 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-005l-2960000000-4aff8e96600a8dcc0cd7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0090000000-1f5244541851cecc3419 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000i-0090000000-ea661d63d95f0276ff50 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-017i-0190000000-bbc4b50b5f9509e31d35 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0090000000-33b322e33c44f750998f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-0090000000-a157f4737fa76857bd9f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-08g4-2960000000-aa5279093d72dcc7f374 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0013960 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 8622316 |
|---|
| ChEBI ID | 308602 |
|---|
| PubChem Compound ID | 10446897 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|