| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:17:49 UTC |
|---|
| Update Date | 2016-11-09 01:17:24 UTC |
|---|
| Accession Number | CHEM022008 |
|---|
| Identification |
|---|
| Common Name | 3'-Hydroxyhexobarbital |
|---|
| Class | Small Molecule |
|---|
| Description | 3'-Hydroxyhexobarbital is only found in individuals that have used or taken Hexobarbital. 3'-Hydroxyhexobarbital is a metabolite of Hexobarbital. 3'-hydroxyhexobarbital belongs to the family of Barbituric Acid Derivatives. These are compounds containing a perhydropyrimidine ring substituted at C-2, -4 and -6 by oxo groups. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
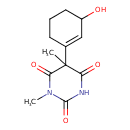
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3'-Hydroxyhexobarbital, (R-(r*,s*))-isomer | HMDB | | 3'-Hydroxyhexobarbital, (r*,s*)-(+-)-isomer | HMDB | | 3'-Hydroxyhexobarbital, (S-(r*,r*))-isomer | HMDB | | 3'-Hydroxyhexobarbital, (S-(r*,s*))-isomer | HMDB | | 3'-Hydroxyhexobarbitone | HMDB | | 3'-Hydroxyhexobarbital, (r*,r*-(+-))-isomer | HMDB | | 3'-Hydroxyhexobarbital, (R-(r*,r*))-isomer | HMDB | | 3'-Hydroxyhexobarbital | MeSH |
|
|---|
| Chemical Formula | C12H16N2O4 |
|---|
| Average Molecular Mass | 252.266 g/mol |
|---|
| Monoisotopic Mass | 252.111 g/mol |
|---|
| CAS Registry Number | 427-29-2 |
|---|
| IUPAC Name | 5-(3-hydroxycyclohex-1-en-1-yl)-1,5-dimethyl-1,3-diazinane-2,4,6-trione |
|---|
| Traditional Name | 5-(3-hydroxycyclohex-1-en-1-yl)-1,5-dimethyl-1,3-diazinane-2,4,6-trione |
|---|
| SMILES | CN1C(=O)NC(=O)C(C)(C2=CC(O)CCC2)C1=O |
|---|
| InChI Identifier | InChI=1S/C12H16N2O4/c1-12(7-4-3-5-8(15)6-7)9(16)13-11(18)14(2)10(12)17/h6,8,15H,3-5H2,1-2H3,(H,13,16,18) |
|---|
| InChI Key | YHCGILGEMWNROZ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as barbituric acid derivatives. Barbituric acid derivatives are compounds containing a perhydropyrimidine ring substituted at C-2, -4 and -6 by oxo groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Diazines |
|---|
| Sub Class | Pyrimidines and pyrimidine derivatives |
|---|
| Direct Parent | Barbituric acid derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Barbiturate
- N-acyl urea
- Ureide
- 1,3-diazinane
- Dicarboximide
- Urea
- Secondary alcohol
- Carbonic acid derivative
- Carboxylic acid derivative
- Azacycle
- Alcohol
- Organonitrogen compound
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Carbonyl group
- Organic nitrogen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001d-6690000000-05c18e141ad01a2adfd5 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-062i-9473000000-3374ccd359522e2377a7 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f79-0090000000-d3579f75324ff06a24ad | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f8i-1940000000-84b97a4011e40fccdaa2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fr6-9210000000-e03f70ca07d3170581db | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0pbc-4290000000-ab688bc86fcdc5eba017 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a59-6900000000-2ae667abb94e9cd35eaf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9200000000-8982cefd0998a1157f87 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0390000000-87f2b4c898dc17fb4c05 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0pdr-1940000000-69d47b739f3406aeb0e8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a6r-3900000000-3696f584e9062a652dbd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0090000000-e9b486d85964be835f03 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fkc-1950000000-36c968a2e66267914088 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9400000000-d7803e836a7cc1b7f497 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0013940 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 141004 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 160460 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|