| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:17:38 UTC |
|---|
| Update Date | 2016-11-09 01:17:24 UTC |
|---|
| Accession Number | CHEM021995 |
|---|
| Identification |
|---|
| Common Name | 8-Hydroxynevirapine |
|---|
| Class | Small Molecule |
|---|
| Description | 8-Hydroxynevirapine is only found in individuals that have used or taken Nevirapine. 8-Hydroxynevirapine is a metabolite of Nevirapine. 8-hydroxynevirapine belongs to the family of 1,4-Diazepines. These are organic compounds containing 1,4-diazepine, a seven-member heterocyclic ring with two nitrogen atoms in positions 1 and 4. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
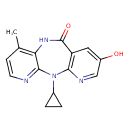
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C15H14N4O2 |
|---|
| Average Molecular Mass | 282.297 g/mol |
|---|
| Monoisotopic Mass | 282.112 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 2-cyclopropyl-13-hydroxy-7-methyl-2,4,9,15-tetraazatricyclo[9.4.0.0³,⁸]pentadeca-1(11),3,5,7,12,14-hexaen-10-one |
|---|
| Traditional Name | 2-cyclopropyl-13-hydroxy-7-methyl-2,4,9,15-tetraazatricyclo[9.4.0.0³,⁸]pentadeca-1(11),3,5,7,12,14-hexaen-10-one |
|---|
| SMILES | CC1=C2NC(=O)C3=C(N=CC(O)=C3)N(C3CC3)C2=NC=C1 |
|---|
| InChI Identifier | InChI=1S/C15H14N4O2/c1-8-4-5-16-14-12(8)18-15(21)11-6-10(20)7-17-13(11)19(14)9-2-3-9/h4-7,9,20H,2-3H2,1H3,(H,18,21) |
|---|
| InChI Key | DZPVEPLRIKDBFC-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as alkyldiarylamines. These are tertiary alkylarylamines having two aryl and one alkyl groups attached to the amino group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic nitrogen compounds |
|---|
| Class | Organonitrogen compounds |
|---|
| Sub Class | Amines |
|---|
| Direct Parent | Alkyldiarylamines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alkyldiarylamine
- Pyrido-para-diazepine
- Para-diazepine
- Methylpyridine
- Hydroxypyridine
- Imidolactam
- Pyridine
- Heteroaromatic compound
- Vinylogous amide
- Carboxamide group
- Lactam
- Secondary carboxylic acid amide
- Carboxylic acid derivative
- Azacycle
- Organoheterocyclic compound
- Organic oxygen compound
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0gyd-1890000000-3cfd1974629bed42b767 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-002k-4869000000-743bd151da9f4be4e422 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0090000000-4db90e3c8eec102dc152 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-1090000000-6ea3809a97c81c186d3b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-002f-9170000000-3221a4376c5aba7ebc60 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0090000000-083099581e2882f2398a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001l-0090000000-84dc076d6a97aa601ae1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05bg-1490000000-58b85aee49066a9e29e2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0090000000-d72761c0346bc09759b0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-0090000000-4b8c21e2532f34918702 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03di-1490000000-e98fafeba7360778c678 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0090000000-7c900f9f8b3f17914393 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001l-0090000000-f426dd3b9af984da0078 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-0190000000-ab9dbb520cbde3aefc95 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0013913 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 8930647 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 10755325 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|