| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:17:23 UTC |
|---|
| Update Date | 2016-11-09 01:17:24 UTC |
|---|
| Accession Number | CHEM021983 |
|---|
| Identification |
|---|
| Common Name | E-10-Hydroxynortriptyline |
|---|
| Class | Small Molecule |
|---|
| Description | E-10-Hydroxynortriptyline is only found in individuals that have used or taken Amitriptyline. E-10-Hydroxynortriptyline is a metabolite of Amitriptyline. E-10-hydroxynortriptyline belongs to the family of Dibenzocycloheptenes. These are compounds containing a dibenzocycloheptene moiety, which consists of two benzene connected by a cycloheptene ring. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
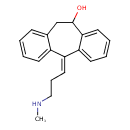
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 10-Hydroxynortriptyline | HMDB | | 10-Hydroxynortriptyline maleate (1:1) | HMDB | | 10-Hydroxynortriptyline hydrochloride | HMDB | | 10-Hydroxynortriptyline, (+-)-isomer | HMDB | | 10-Hydroxynortriptyline, (e)-isomer | HMDB | | 10-Hydroxynortriptyline, (Z)-isomer | HMDB |
|
|---|
| Chemical Formula | C19H21NO |
|---|
| Average Molecular Mass | 279.376 g/mol |
|---|
| Monoisotopic Mass | 279.162 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (2E)-2-[3-(methylamino)propylidene]tricyclo[9.4.0.0³,⁸]pentadeca-1(15),3,5,7,11,13-hexaen-9-ol |
|---|
| Traditional Name | (2E)-2-[3-(methylamino)propylidene]tricyclo[9.4.0.0³,⁸]pentadeca-1(15),3,5,7,11,13-hexaen-9-ol |
|---|
| SMILES | CNCC\C=C1/C2=CC=CC=C2CC(O)C2=CC=CC=C12 |
|---|
| InChI Identifier | InChI=1S/C19H21NO/c1-20-12-6-11-16-15-8-3-2-7-14(15)13-19(21)18-10-5-4-9-17(16)18/h2-5,7-11,19-21H,6,12-13H2,1H3/b16-11+ |
|---|
| InChI Key | VAGXZGJKNUNLHK-LFIBNONCSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dibenzocycloheptenes. Dibenzocycloheptenes are compounds containing a dibenzocycloheptene moiety, which consists of two benzene rings connected by a cycloheptene ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Dibenzocycloheptenes |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Dibenzocycloheptenes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Dibenzocycloheptene
- Secondary alcohol
- Secondary amine
- Secondary aliphatic amine
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Amine
- Alcohol
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01ox-6190000000-6bdfc002b8164f38a92c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0006-9033000000-6a9e01111b58310ed9e0 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01q9-0090000000-1ca30bf7f4a64fbff1af | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01q9-2090000000-bd2d6746902ad67f5563 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052f-4290000000-b4df1745e201740cfb55 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0090000000-a23f7b604598473d807d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0090000000-0e2b231634f3bedce478 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000y-5090000000-17ebb38f8c1ca1a708ee | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0090000000-72bd97d6e51f59b35b9e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-0090000000-f5bea9330037bfa19f74 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-1290000000-0c74e557da6ec34f303a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0090000000-345ef089afddc416f7fc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-06w9-0090000000-34b91c92d338a43242c4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-1190000000-5103dd9b9878439ab144 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0013889 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 4944825 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 6440567 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|