| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:17:11 UTC |
|---|
| Update Date | 2016-11-09 01:17:24 UTC |
|---|
| Accession Number | CHEM021972 |
|---|
| Identification |
|---|
| Common Name | cis,trans-5'-Hydroxythalidomide |
|---|
| Class | Small Molecule |
|---|
| Description | cis,trans-5'-Hydroxythalidomide is only found in individuals that have used or taken Thalidomide. cis,trans-5'-Hydroxythalidomide is a metabolite of Thalidomide. Cis,trans-5'-hydroxythalidomide belongs to the family of Isoindolones. These are aromatic polycyclic compounds that contain an isolindole bearing a ketone. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
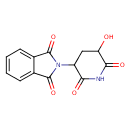
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 5'-OH-Thalidomide | HMDB | | 5'-Hydroxythalidomide | MeSH |
|
|---|
| Chemical Formula | C13H10N2O5 |
|---|
| Average Molecular Mass | 274.229 g/mol |
|---|
| Monoisotopic Mass | 274.059 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 2-(5-hydroxy-2,6-dioxopiperidin-3-yl)-2,3-dihydro-1H-isoindole-1,3-dione |
|---|
| Traditional Name | 2-(5-hydroxy-2,6-dioxopiperidin-3-yl)isoindole-1,3-dione |
|---|
| SMILES | OC1CC(N2C(=O)C3=CC=CC=C3C2=O)C(=O)NC1=O |
|---|
| InChI Identifier | InChI=1S/C13H10N2O5/c16-9-5-8(10(17)14-11(9)18)15-12(19)6-3-1-2-4-7(6)13(15)20/h1-4,8-9,16H,5H2,(H,14,17,18) |
|---|
| InChI Key | HHTOWVWIVBSOKC-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phthalimides. These are aromatic heterocyclic compounds containing a 1,3-dioxoisoindoline moiety. They are imide derivatives of phthalic anhydrides. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Isoindoles and derivatives |
|---|
| Sub Class | Isoindolines |
|---|
| Direct Parent | Phthalimides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phthalimide
- Alpha-amino acid or derivatives
- Isoindole
- Piperidinedione
- Delta-lactam
- Piperidinone
- Benzenoid
- Piperidine
- Carboxylic acid imide, n-substituted
- Carboxylic acid imide
- Dicarboximide
- Carboxylic acid imide, n-unsubstituted
- Lactam
- Secondary alcohol
- Carboxylic acid derivative
- Azacycle
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Alcohol
- Carbonyl group
- Organooxygen compound
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05fr-3960000000-0d3d37ff76fdfb87791e | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00di-4963000000-5c3650820ae538f05811 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0090000000-079837f6d6cfe874a480 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0kdi-0390000000-b2931b26f95e0d654ba2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-05g1-7910000000-127e1af15d9ce6980642 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00ec-3190000000-252159f1998f82fd39dc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05fu-3590000000-daefbb2dfdeb1a8bc739 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0007-9200000000-4897f0c5d2567b205009 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00dj-0790000000-cf25d8d92906fb5756db | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00dj-2980000000-2cd101c0bcf2b3350bb5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-3900000000-fb7da6edc3222a7defa5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0190000000-60d396dd0f7677a62d71 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0690000000-76bf4597a518ab5a1539 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-2900000000-fdf792b17831511bef06 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0013870 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 8054323 |
|---|
| ChEBI ID | 212535 |
|---|
| PubChem Compound ID | 9878646 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|