| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:17:06 UTC |
|---|
| Update Date | 2016-11-09 01:17:24 UTC |
|---|
| Accession Number | CHEM021968 |
|---|
| Identification |
|---|
| Common Name | AFN911 |
|---|
| Class | Small Molecule |
|---|
| Description | AFN911 is a cytochrome P450 (CYP2D6) metabolite of Imatinib. Imatinib is a tyrosine kinase inhibitor, which is used to treat chronic myelogenous leukemia (CML) with BCR-ABL activity, gastrointestinal stromal tumors and acute lymphocytic leukemia (ALL). AFN911 is a 2-phenyl amino pyrimidine derivative. AFN911 is only found in individuals who have consumed or received the drug Imatinib. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
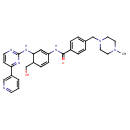
| Chemical Structure | |
|---|
| Synonyms | Not Available |
|---|
| Chemical Formula | C29H33N7O2 |
|---|
| Average Molecular Mass | 511.618 g/mol |
|---|
| Monoisotopic Mass | 511.270 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | N-[4-(hydroxymethyl)-3-{[4-(pyridin-3-yl)pyrimidin-2-yl]amino}cyclohexa-1,5-dien-1-yl]-4-[(4-methylpiperazin-1-yl)methyl]benzamide |
|---|
| Traditional Name | N-[4-(hydroxymethyl)-3-{[4-(pyridin-3-yl)pyrimidin-2-yl]amino}cyclohexa-1,5-dien-1-yl]-4-[(4-methylpiperazin-1-yl)methyl]benzamide |
|---|
| SMILES | CN1CCN(CC2=CC=C(C=C2)C(=O)NC2=CC(NC3=NC=CC(=N3)C3=CN=CC=C3)C(CO)C=C2)CC1 |
|---|
| InChI Identifier | InChI=1S/C29H33N7O2/c1-35-13-15-36(16-14-35)19-21-4-6-22(7-5-21)28(38)32-25-9-8-24(20-37)27(17-25)34-29-31-12-10-26(33-29)23-3-2-11-30-18-23/h2-12,17-18,24,27,37H,13-16,19-20H2,1H3,(H,32,38)(H,31,33,34) |
|---|
| InChI Key | JBSTUPHUZMAVFU-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyridinylpyrimidines. Pyridinylpyrimidines are compounds containing a pyridinylpyrimidine skeleton, which consists of a pyridine linked (not fused) to a pyrimidine by a bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Diazines |
|---|
| Sub Class | Pyrimidines and pyrimidine derivatives |
|---|
| Direct Parent | Pyridinylpyrimidines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyridinylpyrimidine
- Benzamide
- Benzoic acid or derivatives
- Benzoyl
- Benzylamine
- Phenylmethylamine
- Aminopyrimidine
- Aralkylamine
- Secondary aliphatic/aromatic amine
- N-alkylpiperazine
- N-methylpiperazine
- Monocyclic benzene moiety
- 1,4-diazinane
- Piperazine
- Pyridine
- Benzenoid
- Heteroaromatic compound
- Tertiary aliphatic amine
- Tertiary amine
- Secondary carboxylic acid amide
- Carboxamide group
- Amino acid or derivatives
- Carboxylic acid derivative
- Secondary amine
- Azacycle
- Organonitrogen compound
- Amine
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Alcohol
- Organic oxygen compound
- Organooxygen compound
- Primary alcohol
- Organic nitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-030s-4550900000-a183b3890751ee842efe | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-01b9-9432360000-4daad6d13a45784a1bd6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03dl-0141950000-5784bff626ddb7959af2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0296-0493600000-6f52d14414befcadd87b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-02mi-2920000000-6d51f5ab910b8f9ff567 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-1110590000-d515217669cc9b42ffd8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-5332920000-8086a009c4f1b43406bf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-006y-9420100000-a6e983614b417e6cf1bf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-0000390000-90243c608f82e7d2e717 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-0113930000-cef44672a5bfb1573386 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0229-2902410000-cce3695aec9b083ff733 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0000090000-1672730a1d9fa7c204dd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03ec-0017950000-3fd675677f347f8f1b44 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01qi-1301900000-35cc55d1728740c22916 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0013863 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 35032774 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 131750717 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|