| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:17:05 UTC |
|---|
| Update Date | 2016-11-09 01:17:24 UTC |
|---|
| Accession Number | CHEM021967 |
|---|
| Identification |
|---|
| Common Name | 4-Hydroxyifosfamide |
|---|
| Class | Small Molecule |
|---|
| Description | 4-Hydroxyifosfamide is the active metabolite of the bifunctional alkylating cytostatic drug known as ifosfamide. 4-Hydroxyifosfamide is a member of the compound class known as oxazaphosphorines. Oxazaphosphorines are any saturated six-membered heterocycle containing three carbon atoms and one each of oxygen, nitrogen and phosphorus, especially one in which the phosphorus atom is linked to both the nitrogen and oxygen atoms. 4-Hydroxyifosfamide is very unstable in plasma and a stabilization procedure by adding citric acid has been developed (PMID: 9172103). 4-Hydroxyifosfamide is known to pass through the blood-brain barrier, and can reach cerebrospinal fluid concentrations that are almost as high as plasma concentrations (PMID: 9677448). 4-Hydroxyifosfamide is only found in individuals who have consumed or received the drug Ifosfamide. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4-Hydroxy-ifosfamide | HMDB | | 3-(2-Chloroethyl)-2-((2-chloroethyl)amino)-1,3,2-oxazaphosphinan-4-ol 2-oxide | HMDB |
|
|---|
| Chemical Formula | C7H15Cl2N2O3P |
|---|
| Average Molecular Mass | 277.085 g/mol |
|---|
| Monoisotopic Mass | 276.020 g/mol |
|---|
| CAS Registry Number | 67292-64-2 |
|---|
| IUPAC Name | 3-(2-chloroethyl)-2-[(2-chloroethyl)amino]-4-hydroxy-1,3,2λ⁵-oxazaphosphinan-2-one |
|---|
| Traditional Name | 4-hydroxyifosfamide |
|---|
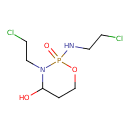
| SMILES | OC1CCOP(=O)(NCCCl)N1CCCl |
|---|
| InChI Identifier | InChI=1S/C7H15Cl2N2O3P/c8-2-4-10-15(13)11(5-3-9)7(12)1-6-14-15/h7,12H,1-6H2,(H,10,13) |
|---|
| InChI Key | JHUJMHKRHQPBRG-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as isofamides. These are oxazaphospholanes containing the isofamide skeleton. Isofamide is a heterocyclic compound made up of a 1,3,2-oxazaphospholane, where the phosphorus atom is part of a phosphodiamide group, and the oxazaphospholane is substituted by two haloalkyl chains. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Oxazaphosphinanes |
|---|
| Sub Class | Isofamides |
|---|
| Direct Parent | Isofamides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Isofamide
- Phosphoric monoester diamide
- Organic phosphoric acid derivative
- Organic phosphoric acid amide
- Alkanolamine
- Azacycle
- Oxacycle
- Organic oxide
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organochloride
- Organic oxygen compound
- Organohalogen compound
- Organic nitrogen compound
- Alkyl halide
- Alkyl chloride
- Hydrocarbon derivative
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-054o-4490000000-68b33a81b7ed61042169 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-00dj-9477000000-c358b806368970e28e8a | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-9030000000-125f3622a612b6133e45 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-9100000000-2dc6532567c6dd55b170 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4l-9000000000-ba299292144772f345bf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03fr-2790000000-5f30604369be13b4f4b1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0089-8900000000-4377372163ecc070ea4d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002f-9000000000-1212b3cc3fd363e6eef8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0090000000-6c1af332aad878673a4b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0290000000-939a59c6fbceef5cc6af | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00bi-3910000000-478bad8cbcb2e8e42d36 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0090000000-368a494088668b022c36 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-2390000000-2745c533925b00075ccd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-9300000000-1dd7c346907cc962b5f5 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0013861 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 272429 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 308171 |
|---|
| Kegg Compound ID | C16553 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|