| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:17:04 UTC |
|---|
| Update Date | 2016-11-09 01:17:24 UTC |
|---|
| Accession Number | CHEM021966 |
|---|
| Identification |
|---|
| Common Name | 2-Dechloroethylifosfamide |
|---|
| Class | Small Molecule |
|---|
| Description | 2-Dechloroethylifosfamide is a metabolite of the antitumor, alkylating drug Ifosfamide. 2-dechloroethylifosfamide is a member of the compound class known as oxazaphosphorines. Oxazaphosphorines are any saturated six-membered heterocycle containing three carbon atoms and one each of oxygen, nitrogen and phosphorus, especially one in which the phosphorus atom is linked to both the nitrogen and oxygen atoms. 2-dechloroethylifosfamide can be biosynthesized from ifosfamide through the action of cytochrome P450 enzymes including CYP3A4, CYP3A5, and CYP2B6 (PMID: 15875221). 2-dechloroethylifosfamide is only found in individuals who have consumed or received the drug Ifosfamide. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
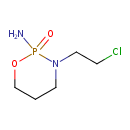
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-DClIF | HMDB | | 2-DCE-iff | HMDB | | 2-Amino-3-(2-chloroethyl)tetrahydro-2H-1,3,2-oxazophosphorine-2-O-xide | HMDB | | Dechloroethylifosfamide, (+-)-isomer | HMDB | | Dechloroethylifosfamide, (R)-isomer | HMDB | | Dechloroethylifosfamide, (S)-isomer | HMDB | | Dechloroethylifosfamide | HMDB |
|
|---|
| Chemical Formula | C5H12ClN2O2P |
|---|
| Average Molecular Mass | 198.588 g/mol |
|---|
| Monoisotopic Mass | 198.032 g/mol |
|---|
| CAS Registry Number | 53459-55-5 |
|---|
| IUPAC Name | 2-amino-3-(2-chloroethyl)-1,3,2λ⁵-oxazaphosphinan-2-one |
|---|
| Traditional Name | dechloroethylifosfamide |
|---|
| SMILES | NP1(=O)OCCCN1CCCl |
|---|
| InChI Identifier | InChI=1S/C5H12ClN2O2P/c6-2-4-8-3-1-5-10-11(8,7)9/h1-5H2,(H2,7,9) |
|---|
| InChI Key | ROGLJLJCDSTWBN-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phosphoric monoester diamides. These are organophosphorus compounds containing a monoamide derivative of a phosphoric acid diester functional group. They have the general structure R1OP(=O)(N(R2)R3)N(R4)R5, where R1 = organyl group and R2-R5 = H or organyl. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Organic phosphoric acids and derivatives |
|---|
| Sub Class | Organic phosphoramides |
|---|
| Direct Parent | Phosphoric monoester diamides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phosphoric monoester diamide
- Oxazaphosphinane
- Organoheterocyclic compound
- Azacycle
- Oxacycle
- Alkyl chloride
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Organochloride
- Organohalogen compound
- Alkyl halide
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-03g1-5900000000-0197e550072c40b50073 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-9600000000-d7aaece789b967cf6d49 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01wf-7900000000-5e5fda28174d2f38e034 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9100000000-f851ecebf6b0ccd4c523 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-2900000000-ecfabfb83bc9e1fffb60 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-7900000000-8ef68b55534d7b1f386e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-004i-9000000000-14e8ca9900631824076f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0900000000-30ab82031d48f66f45ce | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0900000000-4573dc12b33bcf32df76 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-9100000000-bd289a1212000ffd9b33 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-d67a78722745354ea944 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-5900000000-baad3afa942ca9d191c2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01t9-9200000000-81ded39078c95bc7eeba | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0013859 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 106420 |
|---|
| ChEBI ID | 80562 |
|---|
| PubChem Compound ID | 119105 |
|---|
| Kegg Compound ID | C16555 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|