| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:17:01 UTC |
|---|
| Update Date | 2016-11-09 01:17:24 UTC |
|---|
| Accession Number | CHEM021964 |
|---|
| Identification |
|---|
| Common Name | 4-Hydroxycyclophosphamide |
|---|
| Class | Small Molecule |
|---|
| Description | A phosphorodiamide that consists of 2-amino-1,3,2-oxazaphosphinan-4-ol 2-oxide having two 2-chloroethyl groups attached to the exocyclic nitrogen. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
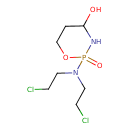
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Tetrahydro-2-(bis(2-chloroethyl)amino)-2H-1,3,2-oxazaphosphorin-4-ol 2-oxide | ChEBI | | 4-Hydroxycyclophosphamide, (cis)-isomer | HMDB | | 4-Hydroxycyclophosphamide, (trans)-isomer | HMDB |
|
|---|
| Chemical Formula | C7H15Cl2N2O3P |
|---|
| Average Molecular Mass | 277.085 g/mol |
|---|
| Monoisotopic Mass | 276.020 g/mol |
|---|
| CAS Registry Number | 40277-05-2 |
|---|
| IUPAC Name | 2-[bis(2-chloroethyl)amino]-4-hydroxy-1,3,2λ⁵-oxazaphosphinan-2-one |
|---|
| Traditional Name | 4-hydroxycyclophosphamide |
|---|
| SMILES | OC1CCOP(=O)(N1)N(CCCl)CCCl |
|---|
| InChI Identifier | InChI=1S/C7H15Cl2N2O3P/c8-2-4-11(5-3-9)15(13)10-7(12)1-6-14-15/h7,12H,1-6H2,(H,10,13) |
|---|
| InChI Key | RANONBLIHMVXAJ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as nitrogen mustard compounds. Nitrogen mustard compounds are compounds having two beta-haloalkyl groups bound to a nitrogen atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic nitrogen compounds |
|---|
| Class | Organonitrogen compounds |
|---|
| Sub Class | Nitrogen mustard compounds |
|---|
| Direct Parent | Nitrogen mustard compounds |
|---|
| Alternative Parents | |
|---|
| Substituents | - Nitrogen mustard
- Phosphoric monoester diamide
- Organic phosphoric acid derivative
- Oxazaphosphinane
- Organic phosphoric acid amide
- Azacycle
- Organoheterocyclic compound
- Alkanolamine
- Oxacycle
- Alkyl chloride
- Organopnictogen compound
- Organooxygen compound
- Organochloride
- Organohalogen compound
- Organic oxygen compound
- Alkyl halide
- Hydrocarbon derivative
- Organic oxide
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-02bu-5890000000-a427d5c4bf9209482b43 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-05g1-7392000000-4b076778aadb22b57ed1 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a6r-8290000000-b01da8a61b60048b29f7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-002f-9800000000-70f292b6f3c854cf93a2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a6u-9000000000-c8f75a918284b672a062 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03fr-1790000000-e9e004489e6cc1b1144d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-9000000000-9b317f1b0c85a67d882e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0007-9000000000-21ced355676eb4ed7c49 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0090000000-6c1af332aad878673a4b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-1290000000-097d11cc59c075d92e8e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01u0-9100000000-358d49458e7399802e5d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0090000000-312fa45820b98bd8a809 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-3790000000-737d73cd2cdf34a3c57e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4l-9520000000-a847f1236ae0f8522c8a | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0013856 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | 4-Hydroxycyclophosphamide |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 1864 |
|---|
| PubChem Compound ID | 99735 |
|---|
| Kegg Compound ID | C07643 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|