| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:16:57 UTC |
|---|
| Update Date | 2016-11-09 01:17:24 UTC |
|---|
| Accession Number | CHEM021961 |
|---|
| Identification |
|---|
| Common Name | 4-Hydroxyvalsartan |
|---|
| Class | Small Molecule |
|---|
| Description | 4-Hydroxyvalsartan is only found in individuals that have used or taken Valsartan. 4-Hydroxyvalsartan is a metabolite of Valsartan. 4-hydroxyvalsartan belongs to the family of Biphenyltetrazoles and Derivatives. These are organic compounds containing a biphenyl attached to a tetrazole. A carbon atom of the biphenyl moiety is boned to a carbon or the nitrogen atom of the tetrazole moiety. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
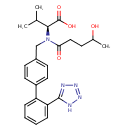
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (2S)-2-(4-Hydroxy-N-{[2'-(2H-1,2,3,4-tetrazol-5-yl)-[1,1'-biphenyl]-4-yl]methyl}pentanamido)-3-methylbutanoate | Generator |
|
|---|
| Chemical Formula | C24H29N5O4 |
|---|
| Average Molecular Mass | 451.518 g/mol |
|---|
| Monoisotopic Mass | 451.222 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | (2S)-2-[4-hydroxy-N-({4-[2-(1H-1,2,3,4-tetrazol-5-yl)phenyl]phenyl}methyl)pentanamido]-3-methylbutanoic acid |
|---|
| Traditional Name | (2S)-2-[4-hydroxy-N-({4-[2-(1H-1,2,3,4-tetrazol-5-yl)phenyl]phenyl}methyl)pentanamido]-3-methylbutanoic acid |
|---|
| SMILES | CC(O)CCC(=O)N(CC1=CC=C(C=C1)C1=CC=CC=C1C1=NN=NN1)[C@@H](C(C)C)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C24H29N5O4/c1-15(2)22(24(32)33)29(21(31)13-8-16(3)30)14-17-9-11-18(12-10-17)19-6-4-5-7-20(19)23-25-27-28-26-23/h4-7,9-12,15-16,22,30H,8,13-14H2,1-3H3,(H,32,33)(H,25,26,27,28)/t16?,22-/m0/s1 |
|---|
| InChI Key | ICSQZMPILLPFKC-XLDIYJRPSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as valine and derivatives. Valine and derivatives are compounds containing valine or a derivative thereof resulting from reaction of valine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Valine and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - N-acyl-alpha-amino acid
- Valine or derivatives
- N-acyl-alpha amino acid or derivatives
- N-acyl-l-alpha-amino acid
- Biphenyl
- Phenyltetrazole
- Monocyclic benzene moiety
- N-acyl-amine
- Benzenoid
- Azole
- Heteroaromatic compound
- Tertiary carboxylic acid amide
- Tetrazole
- Secondary alcohol
- Carboxamide group
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Azacycle
- Organoheterocyclic compound
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Carbonyl group
- Organic oxygen compound
- Alcohol
- Organic nitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000i-4192300000-95389c126057ce193ac5 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-00di-9101230000-8a2c579928e8abbfef42 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f8i-0032900000-090a3f00221357e7ad51 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-3198700000-08d3a5c412202f745eca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-3293000000-6e0d1aa663fbf73da0a4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0zfr-0002900000-394299cf885ddb6ab69e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0zfr-2219800000-3450a6cb70e11f7ec61b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-02mj-3916000000-4953d84593a718a96ad3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0uea-9000500000-4f74cafb2b4ad511d352 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0gc1-4629100000-f7e8f5e1c194841e70f6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-02t9-5891000000-ecc03346e3445d008848 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f79-0094600000-5c615ff5543c2efc86f0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0019-0096000000-326ae10f841aeaa2e2e0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-0191000000-57491a9983bd0556909d | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0013848 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 8087298 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 9911647 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|