| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:16:56 UTC |
|---|
| Update Date | 2016-11-09 01:17:24 UTC |
|---|
| Accession Number | CHEM021959 |
|---|
| Identification |
|---|
| Common Name | E-3179 |
|---|
| Class | Small Molecule |
|---|
| Description | E-3179 is a metabolite of Losartan. E-3179 belongs to the family of Anilides. These are organic compounds derived from oxoacids RkE(=O)l(OH)m by replacing an OH group by the NHPh group or derivative formed by ring substitution. |
|---|
| Contaminant Sources | - HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
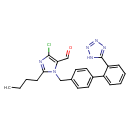
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| EXP 132 | HMDB | | 2-Butyl-4-chloro-1-((2'-(1H-tetrazol-5-yl)(1,1'-biphenyl)-4-yl)methyl)-1H-imidazole-5-carboxaldehyde | HMDB | | EXP-132 | HMDB |
|
|---|
| Chemical Formula | C22H21ClN6O |
|---|
| Average Molecular Mass | 420.895 g/mol |
|---|
| Monoisotopic Mass | 420.147 g/mol |
|---|
| CAS Registry Number | Not Available |
|---|
| IUPAC Name | 2-butyl-4-chloro-1-({4-[2-(1H-1,2,3,4-tetrazol-5-yl)phenyl]phenyl}methyl)-1H-imidazole-5-carbaldehyde |
|---|
| Traditional Name | 2-butyl-5-chloro-3-({4-[2-(1H-1,2,3,4-tetrazol-5-yl)phenyl]phenyl}methyl)imidazole-4-carbaldehyde |
|---|
| SMILES | CCCCC1=NC(Cl)=C(C=O)N1CC1=CC=C(C=C1)C1=CC=CC=C1C1=NN=NN1 |
|---|
| InChI Identifier | InChI=1S/C22H21ClN6O/c1-2-3-8-20-24-21(23)19(14-30)29(20)13-15-9-11-16(12-10-15)17-6-4-5-7-18(17)22-25-27-28-26-22/h4-7,9-12,14H,2-3,8,13H2,1H3,(H,25,26,27,28) |
|---|
| InChI Key | FQZSMTSTFMNWQF-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as biphenyls and derivatives. These are organic compounds containing to benzene rings linked together by a C-C bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Biphenyls and derivatives |
|---|
| Direct Parent | Biphenyls and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Biphenyl
- Phenyltetrazole
- 1,2,4,5-tetrasubstituted imidazole
- Imidazole-4-carbonyl group
- Aryl-aldehyde
- Aryl chloride
- Aryl halide
- N-substituted imidazole
- Azole
- Heteroaromatic compound
- Imidazole
- Vinylogous halide
- Tetrazole
- Azacycle
- Organoheterocyclic compound
- Organohalogen compound
- Organooxygen compound
- Aldehyde
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organochloride
- Organonitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-002u-4189000000-b97a11ba828d83b9a63f | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0021900000-92e20648517914146b50 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0193100000-3e8bd99e0968b8199adf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03dr-9161000000-e4a6d24a60a1c7409a3f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0301900000-5217a4057e4e712eb92a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-002r-0907300000-72743a54570ba14ec469 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-002b-3901000000-bfac8427e2fc4607fe8c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0007900000-44bf34d734ef66bb31a2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00lr-7916500000-997effada2a7385187cb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000w-4930000000-6034f7f794bdf3fe9453 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00dr-0050900000-92f01f1155f25fc27aa2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-052r-0290000000-83c0755147fb67e7801f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-0490000000-46437d86d2228e07cafa | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0013846 |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | 4th millennium |
|---|
| Chemspider ID | 148830 |
|---|
| ChEBI ID | 174143 |
|---|
| PubChem Compound ID | 170214 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|