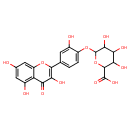| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:16:27 UTC |
|---|
| Update Date | 2016-11-09 01:17:23 UTC |
|---|
| Accession Number | CHEM021945 |
|---|
| Identification |
|---|
| Common Name | Quercetin 4'-glucuronide |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | - FooDB Chemicals
- HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-Hydroxy-4-(3,5,7-trihydroxy-4-oxo-4H-1-benzopyran-2-yl)phenyl beta-D-glucopyranosiduronic acid | HMDB | | Quercetin 4'-glucuronide | HMDB | | Quercetin 4'-O-glucuronide | HMDB | | 3,4,5-Trihydroxy-6-[2-hydroxy-4-(3,5,7-trihydroxy-4-oxo-4H-chromen-2-yl)phenoxy]oxane-2-carboxylate | HMDB |
|
|---|
| Chemical Formula | C21H18O13 |
|---|
| Average Molecular Mass | 478.360 g/mol |
|---|
| Monoisotopic Mass | 478.075 g/mol |
|---|
| CAS Registry Number | 201463-36-7 |
|---|
| IUPAC Name | 3,4,5-trihydroxy-6-[2-hydroxy-4-(3,5,7-trihydroxy-4-oxo-4H-chromen-2-yl)phenoxy]oxane-2-carboxylic acid |
|---|
| Traditional Name | 3,4,5-trihydroxy-6-[2-hydroxy-4-(3,5,7-trihydroxy-4-oxochromen-2-yl)phenoxy]oxane-2-carboxylic acid |
|---|
| SMILES | O[C@@H]1[C@@H](O)[C@H](OC2=CC=C(C=C2O)C2=C(O)C(=O)C3=C(O)C=C(O)C=C3O2)O[C@@H]([C@H]1O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C21H18O13/c22-7-4-9(24)12-11(5-7)32-18(15(27)13(12)25)6-1-2-10(8(23)3-6)33-21-17(29)14(26)16(28)19(34-21)20(30)31/h1-5,14,16-17,19,21-24,26-29H,(H,30,31)/t14-,16-,17+,19-,21+/m0/s1 |
|---|
| InChI Key | OUQZCILJSVDJFB-JENRNSKYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as flavonoid o-glucuronides. These are phenolic compounds containing a flavonoid moiety which is O-glycosidically linked to glucuronic acid. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Flavonoids |
|---|
| Sub Class | Flavonoid glycosides |
|---|
| Direct Parent | Flavonoid O-glucuronides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Flavonoid-4p-o-glucuronide
- 3-hydroxyflavone
- 3'-hydroxyflavonoid
- 3-hydroxyflavonoid
- 5-hydroxyflavonoid
- 7-hydroxyflavonoid
- Flavone
- Hydroxyflavonoid
- Phenolic glycoside
- 1-o-glucuronide
- O-glucuronide
- Glucuronic acid or derivatives
- Chromone
- Glycosyl compound
- O-glycosyl compound
- Benzopyran
- 1-benzopyran
- Phenol ether
- Phenoxy compound
- Phenol
- Pyranone
- 1-hydroxy-2-unsubstituted benzenoid
- Beta-hydroxy acid
- 1-hydroxy-4-unsubstituted benzenoid
- Oxane
- Monocyclic benzene moiety
- Pyran
- Hydroxy acid
- Benzenoid
- Monosaccharide
- Vinylogous acid
- Heteroaromatic compound
- Secondary alcohol
- Monocarboxylic acid or derivatives
- Acetal
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Carboxylic acid derivative
- Carboxylic acid
- Alcohol
- Organooxygen compound
- Hydrocarbon derivative
- Carbonyl group
- Organic oxygen compound
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0bt9-9107300000-7d63fc0eeb1412feb5a9 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0059-3365029000-aabd20466929c11011bb | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0w4i-0117900000-62b27d6da078aec6b615 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0udi-0249100000-dc95a565d440a779770c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0k9i-2942000000-f9bdc2a15871c05ac37a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0fb9-0104900000-72745aae4e41e2f84e98 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-2229400000-04f0fed8bdd2f8fc962e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udi-5966000000-ee0e73ce66484cdecbb5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-0000900000-ccb1d2612c30e5d3fe93 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-0510900000-f6197fa55e8da1db8a0a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0uy0-2953300000-f35820601cb70c0b229d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-0000900000-5bca03d6e246fbc6912f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0000900000-c2ac40badcd40eabda6c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0v00-2910200000-54af6aadfd5a5e7f7902 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0029214 |
|---|
| FooDB ID | FDB031403 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00013836 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 57331045 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|