| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:15:54 UTC |
|---|
| Update Date | 2016-11-09 01:17:23 UTC |
|---|
| Accession Number | CHEM021926 |
|---|
| Identification |
|---|
| Common Name | Malvidin 3-galactoside |
|---|
| Class | Small Molecule |
|---|
| Description | Malvidin 3-galactoside is found in american cranberry. Malvidin 3-galactoside is isolated from many plant species including Vaccinium myrtillus (bilberry) and Vaccinium corymbosum (blueberry) Malvidin (Mv) is an anthocyanidin. As a primary plant pigment, its glycosides are highly abundant in nature. It is primarily responsible for the color of red wine, Vitis vinifera being one of its sources. It is also one of the anthocyanidins responsible for the blue pigment found in the Primula polyanthus plant. Malvidin is an anthocyanin. Anthocyanins are pigments that give color red to red grape (Vitis vinifera) varieties, and blood oranges (Citrus sinensis (L.) Osbeck). (PMID: 15563216, 17425943); Anthocyanins have potentially chemopreventive activity, apart from its antioxidant activity. (PMID: 16080535); Numerous classes of grape anthocyanins are transferred to the wine and confer taste and color to the beverage. (PMID: 15954164); Anthocyanins are water soluble pigments belonging to the flavonoids compound family involved in nature in a wide range of functions such as flowers, fruits, and seeds pigmentation to attract pollinators, to disperse seeds, to protect against UV light damage, and in plant defense to protect against pathogen attack. Because anthocyanins impart much of the color and flavor of fruits and vegetables, they are usually components of the human diet and are not only considered exclusively as food products but also as therapeutic agents; in fact, anthocyanins have been suggested to protect against oxidative stress, coronary heart diseases, certain cancers, and other age-related diseases. At least part of these presumed health-promoting features can be attributed to the antioxidant properties of these compounds whose chemical structure appears ideal for free radical scavenging. (PMID: 16277406). |
|---|
| Contaminant Sources | - FooDB Chemicals
- HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
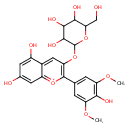
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Malvidin 3-O-beta-D-galactopyranoside | HMDB | | Primulin | HMDB | | Uliginosin | HMDB | | Malvidin 3-galactoside, chloride, (D)-isomer | MeSH | | Malvidin 3-galactoside | MeSH |
|
|---|
| Chemical Formula | C23H25O12 |
|---|
| Average Molecular Mass | 493.437 g/mol |
|---|
| Monoisotopic Mass | 493.135 g/mol |
|---|
| CAS Registry Number | 30113-37-2 |
|---|
| IUPAC Name | 5,7-dihydroxy-2-(4-hydroxy-3,5-dimethoxyphenyl)-3-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-1λ⁴-chromen-1-ylium |
|---|
| Traditional Name | oenin |
|---|
| SMILES | COC1=CC(=CC(OC)=C1O)C1=C(OC2OC(CO)C(O)C(O)C2O)C=C2C(O)=CC(O)=CC2=[O+]1 |
|---|
| InChI Identifier | InChI=1S/C23H24O12/c1-31-14-3-9(4-15(32-2)18(14)27)22-16(7-11-12(26)5-10(25)6-13(11)33-22)34-23-21(30)20(29)19(28)17(8-24)35-23/h3-7,17,19-21,23-24,28-30H,8H2,1-2H3,(H2-,25,26,27)/p+1 |
|---|
| InChI Key | PXUQTDZNOHRWLI-UHFFFAOYSA-O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as anthocyanidin-3-o-glycosides. These are phenolic compounds containing one anthocyanidin moiety which is O-glycosidically linked to a carbohydrate moiety at the C3-position. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Flavonoids |
|---|
| Sub Class | Flavonoid glycosides |
|---|
| Direct Parent | Anthocyanidin-3-O-glycosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Anthocyanidin-3-o-glycoside
- Flavonoid-3-o-glycoside
- 3p-methoxyflavonoid-skeleton
- Hydroxyflavonoid
- 4'-hydroxyflavonoid
- 5-hydroxyflavonoid
- 7-hydroxyflavonoid
- Anthocyanidin
- Hexose monosaccharide
- Glycosyl compound
- O-glycosyl compound
- Benzopyran
- Methoxyphenol
- M-dimethoxybenzene
- 1-benzopyran
- Dimethoxybenzene
- Anisole
- Phenoxy compound
- Phenol ether
- Methoxybenzene
- Phenol
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Oxane
- Monosaccharide
- Monocyclic benzene moiety
- Benzenoid
- Heteroaromatic compound
- Secondary alcohol
- Oxacycle
- Organoheterocyclic compound
- Acetal
- Polyol
- Ether
- Alcohol
- Primary alcohol
- Hydrocarbon derivative
- Organooxygen compound
- Organic oxygen compound
- Organic cation
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05fr-9201700000-5f224aacce0b77a00286 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-05fr-9300028000-6ce1f0efa7ecad1c1395 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0006-0100900000-033710abfed082d2d370 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-1300900000-e69b0ff159f559310723 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-8902100000-743f1ed57444e7ba81c8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-2300900000-395bbc23a2b9330368ce | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-6700900000-535b30c2ac9d26860dd4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0596-9300000000-0d5daf968ba28401b21c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001l-0005900000-76424194529c1604a3b8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001i-1009500000-103645b5940ac4228fb6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-02ai-3039200000-e1c3b9411cfeaed0326d | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0038010 |
|---|
| FooDB ID | FDB017212 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00006734 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Primulin (anthocyanin) |
|---|
| Chemspider ID | 2806159 |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | 3568969 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Hillebrand S, Schwarz M, Winterhalter P: Characterization of anthocyanins and pyranoanthocyanins from blood orange [Citrus sinensis (L.) Osbeck] juice. J Agric Food Chem. 2004 Dec 1;52(24):7331-8. | | 2. Bovell-Benjamin AC: Sweet potato: a review of its past, present, and future role in human nutrition. Adv Food Nutr Res. 2007;52:1-59. | | 3. Fimognari C, Berti F, Nusse M, Cantelli-Fortii G, Hrelia P: In vitro anticancer activity of cyanidin-3-O-beta-glucopyranoside: effects on transformed and non-transformed T lymphocytes. Anticancer Res. 2005 Jul-Aug;25(4):2837-40. | | 4. Flamini R: Some advances in the knowledge of grape, wine and distillates chemistry as achieved by mass spectrometry. J Mass Spectrom. 2005 Jun;40(6):705-13. | | 5. Lo Piero AR, Puglisi I, Rapisarda P, Petrone G: Anthocyanins accumulation and related gene expression in red orange fruit induced by low temperature storage. J Agric Food Chem. 2005 Nov 16;53(23):9083-8. | | 6. Yannai, Shmuel. (2004) Dictionary of food compounds with CD-ROM: Additives, flavors, and ingredients. Boca Raton: Chapman & Hall/CRC. |
|
|---|