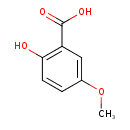
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0v4i-1900000000-1e109eb0ee8ae327f133 | Spectrum |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0092-3290000000-b2c04fbea10000b9034e | Spectrum |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum |
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-02t9-2900000000-eca7c3623a7975e733d7 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-0uk9-5900000000-5aaba4f93b26c36dfcb7 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0fvl-9100000000-94c4137aab8bf06f3617 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - MALDI-TOF (Voyager DE-PRO, Applied Biosystems) , Negative | splash10-0udi-0901000000-077381dba9d80706a097 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - MALDI-TOF (Voyager DE-PRO, Applied Biosystems) , Positive | splash10-0fri-0930000000-4e24b1261f33fba704e2 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - ESI-TOF , Negative | splash10-0a4i-0529000000-a613547802dab4125d68 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - ESI-TOF 20V, Negative | splash10-0a4i-0529000000-a613547802dab4125d68 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - ESI-TOF 10V, Negative | splash10-0a4i-0529000000-a613547802dab4125d68 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - ESI-TOF , Negative | splash10-0a4i-0529000000-a613547802dab4125d68 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - ESI-TOF 20V, Negative | splash10-0a4i-0900000000-e2d69678bf5361774dbf | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - ESI-TOF 10V, Negative | splash10-0pvi-0900000000-46471751a79101b1fd39 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-TOF , negative | splash10-0a4i-0900000000-e2d69678bf5361774dbf | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-TOF , negative | splash10-0pvi-0900000000-46471751a79101b1fd39 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Negative | splash10-0pb9-0900000000-4fe7b9af6ce0405606a6 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0900000000-e2d69678bf5361774dbf | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Positive | splash10-0fdk-9700000000-dcbbb81e035f6deb9148 | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0900000000-844a327be60247da81ae | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 40V, Positive | splash10-0zfr-9200000000-29a841e2d36df5762a0d | Spectrum |
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-1900000000-a9825a2a16be2ec1b6d0 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0gb9-0900000000-7ee52209d923d99f69ef | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0uk9-0900000000-d2f3d14888f1a31ec2c7 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01c1-9300000000-1ba0741166e840d38463 | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-01b9-0900000000-2c570bf0fdb5915da23a | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-05fr-0900000000-77290f37ff3e5699255a | Spectrum |
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-5900000000-b642574365d90b34c12c | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
| 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available | Spectrum |