| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:14:34 UTC |
|---|
| Update Date | 2016-11-09 01:17:23 UTC |
|---|
| Accession Number | CHEM021875 |
|---|
| Identification |
|---|
| Common Name | Angiotensin II |
|---|
| Class | Small Molecule |
|---|
| Description | Angiotensin II is under investigation for the treatment of Sepsis, Septic Shock, Diabetes Mellitus, and Acute Renal Failure. Angiotensin II has been investigated for the treatment, basic science, and diagnostic of Hypertension, Renin Angiotensin System, and Idiopathic Membranous Nephropathy.
As of December 21, 2017 the FDA approved La Jolla Pharmaceutical's Giapreza (angiotensin II) Injection for Intravenouse Infusion for the indication of acting as a vasoconstrictor to increase blood pressure in adults with septic or other distributive shock. The novelty of the medication lies in the fact that it is the first and only use of synthetic Angiotensin II to help maintain body blood pressure.
Shock is the inability to maintain blood flow to vital tissues and the potential resultant organ failure and death within hours, no matter young or o ld. As distributive shock is the most common type of shock in the inpatient setting and affects up to one third of patients in the intensive care unit, the FDA determined that there is a need for treatment options for critically ill hypotensive patients who do not adequately respond to currently available therapies. |
|---|
| Contaminant Sources | - FooDB Chemicals
- HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
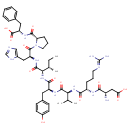
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 5-Isoleucine-angiotensin II | ChEBI | | 5-L-Isoleucineangiotensin II | ChEBI | | Angiotensin | ChEBI | | Angiotensin II (human) | ChEBI | | Angiotensin II (mouse) | ChEBI | | Angiotonin | ChEBI | | Asp-arg-val-tyr-ile-his-pro-phe | ChEBI | | Human angiotensin II | ChEBI | | Hypertensin | ChEBI | | Isoleucine(5)-angiotensin II | ChEBI | | N-(1-(N-(N-(N-(N-(N(2)-L-alpha-Aspartyl-L-arginyl)-L-valyl)-L-tyrosyl)-L-isoleucyl)-L-histidyl)-L-prolyl)-L-phenylalanine | ChEBI | | Delivert | Kegg | | N-(1-(N-(N-(N-(N-(N(2)-L-a-Aspartyl-L-arginyl)-L-valyl)-L-tyrosyl)-L-isoleucyl)-L-histidyl)-L-prolyl)-L-phenylalanine | Generator | | N-(1-(N-(N-(N-(N-(N(2)-L-Α-aspartyl-L-arginyl)-L-valyl)-L-tyrosyl)-L-isoleucyl)-L-histidyl)-L-prolyl)-L-phenylalanine | Generator | | 1-8-Angiotensin I | HMDB | | 1-L-Aspasaginyl-5-L-valyl angiotensin octapeptide | HMDB | | Ang II | HMDB | | Angiotensin 2 | HMDB | | Ile(5)-angiotensin II | HMDB | | II, 5-L-isoleucine angiotensin | HMDB | | Isoleucyl(5)-angiotensin II | HMDB | | Angiotensin II, 5-L-isoleucine | HMDB | | Valyl(5)-angiotensin II | HMDB | | ANG-(1-8)octapeptide | HMDB | | Isoleucine(5)-angiotensin | HMDB | | 5 L Isoleucine angiotensin II | HMDB | | 5-L-Isoleucine angiotensin II | HMDB | | Angiotensin-(1-8) octapeptide | HMDB |
|
|---|
| Chemical Formula | C50H71N13O12 |
|---|
| Average Molecular Mass | 1046.179 g/mol |
|---|
| Monoisotopic Mass | 1045.535 g/mol |
|---|
| CAS Registry Number | 11128-99-7 |
|---|
| IUPAC Name | (3S)-3-amino-3-{[(1S)-1-{[(1S)-1-{[(1S)-1-{[(1S,2S)-1-{[(2S)-1-[(2S)-2-{[(1S)-1-carboxy-2-phenylethyl]carbamoyl}pyrrolidin-1-yl]-3-(1H-imidazol-5-yl)-1-oxopropan-2-yl]carbamoyl}-2-methylbutyl]carbamoyl}-2-(4-hydroxyphenyl)ethyl]carbamoyl}-2-methylpropyl]carbamoyl}-4-[(diaminomethylidene)amino]butyl]carbamoyl}propanoic acid |
|---|
| Traditional Name | (3S)-3-amino-3-{[(1S)-1-{[(1S)-1-{[(1S)-1-{[(1S,2S)-1-{[(2S)-1-[(2S)-2-{[(1S)-1-carboxy-2-phenylethyl]carbamoyl}pyrrolidin-1-yl]-3-(3H-imidazol-4-yl)-1-oxopropan-2-yl]carbamoyl}-2-methylbutyl]carbamoyl}-2-(4-hydroxyphenyl)ethyl]carbamoyl}-2-methylpropyl]carbamoyl}-4-[(diaminomethylidene)amino]butyl]carbamoyl}propanoic acid |
|---|
| SMILES | CC[C@H](C)[C@H](NC(=O)[C@H](CC1=CC=C(O)C=C1)NC(=O)[C@@H](NC(=O)[C@H](CCCN=C(N)N)NC(=O)[C@@H](N)CC(O)=O)C(C)C)C(=O)N[C@@H](CC1=CN=CN1)C(=O)N1CCC[C@H]1C(=O)N[C@@H](CC1=CC=CC=C1)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C50H71N13O12/c1-5-28(4)41(47(72)59-36(23-31-25-54-26-56-31)48(73)63-20-10-14-38(63)45(70)60-37(49(74)75)22-29-11-7-6-8-12-29)62-44(69)35(21-30-15-17-32(64)18-16-30)58-46(71)40(27(2)3)61-43(68)34(13-9-19-55-50(52)53)57-42(67)33(51)24-39(65)66/h6-8,11-12,15-18,25-28,33-38,40-41,64H,5,9-10,13-14,19-24,51H2,1-4H3,(H,54,56)(H,57,67)(H,58,71)(H,59,72)(H,60,70)(H,61,68)(H,62,69)(H,65,66)(H,74,75)(H4,52,53,55)/t28-,33-,34-,35-,36-,37-,38-,40-,41-/m0/s1 |
|---|
| InChI Key | CZGUSIXMZVURDU-JZXHSEFVSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as oligopeptides. These are organic compounds containing a sequence of between three and ten alpha-amino acids joined by peptide bonds. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Oligopeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-oligopeptide
- Tyrosine or derivatives
- Phenylalanine or derivatives
- Histidine or derivatives
- Aspartic acid or derivatives
- Isoleucine or derivatives
- N-acyl-alpha-amino acid
- N-acyl-alpha amino acid or derivatives
- N-acyl-l-alpha-amino acid
- Valine or derivatives
- Proline or derivatives
- Alpha-amino acid amide
- 3-phenylpropanoic-acid
- Alpha-amino acid or derivatives
- N-substituted-alpha-amino acid
- Amphetamine or derivatives
- N-acylpyrrolidine
- Pyrrolidine-2-carboxamide
- Imidazolyl carboxylic acid derivative
- Pyrrolidine carboxylic acid or derivatives
- Phenol
- 1-hydroxy-2-unsubstituted benzenoid
- Monocyclic benzene moiety
- Dicarboxylic acid or derivatives
- Fatty acyl
- Fatty amide
- Benzenoid
- N-acyl-amine
- Pyrrolidine
- Tertiary carboxylic acid amide
- Imidazole
- Heteroaromatic compound
- Azole
- Amino acid or derivatives
- Amino acid
- Carboxamide group
- Guanidine
- Secondary carboxylic acid amide
- Carboximidamide
- Carboxylic acid
- Azacycle
- Organoheterocyclic compound
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Organooxygen compound
- Organopnictogen compound
- Carbonyl group
- Hydrocarbon derivative
- Organic oxygen compound
- Primary amine
- Organic nitrogen compound
- Amine
- Organonitrogen compound
- Organic oxide
- Primary aliphatic amine
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03mj-9313022101-6ac3b05e269115b1f5ad | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-9724022100-1dce7a6f99b2d561c0b3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01p6-9111000000-18aeed5d9ab235b9a89b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0zi3-9000000005-bb2ec7da92fff6a158ac | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-9110001228-620241ce085107b44de3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9510024340-d26443900117133d12ca | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-022d-6194112202-719c98c76d553cec3659 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-5974214021-eb3b74dcc95ba9b408e9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00bi-3900000320-04aedfa1b8f8c426ec9d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-002f-9010000101-5b3797a066dced181aa2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00ai-9214100305-4619422d9477b060e609 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-03di-7252290207-1417dac20cfbdae1d2d1 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | DB11842 |
|---|
| HMDB ID | HMDB0001035 |
|---|
| FooDB ID | FDB022383 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Angiotensin |
|---|
| Chemspider ID | 150504 |
|---|
| ChEBI ID | 2719 |
|---|
| PubChem Compound ID | 172198 |
|---|
| Kegg Compound ID | C02135 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. Malpas SC, Ramchandra R, Guild SJ, McBryde F, Barrett CJ: Renal sympathetic nerve activity in the development of hypertension. Curr Hypertens Rep. 2006 Jun;8(3):242-8. | | 2. Durvasula RV, Shankland SJ: The renin-angiotensin system in glomerular podocytes: mediator of glomerulosclerosis and link to hypertensive nephropathy. Curr Hypertens Rep. 2006 May;8(2):132-8. | | 3. Wassmann S, Nickenig G: Pathophysiological regulation of the AT1-receptor and implications for vascular disease. J Hypertens Suppl. 2006 Mar;24(1):S15-21. | | 4. Ruiz-Ortega M, Esteban V, Ruperez M, Sanchez-Lopez E, Rodriguez-Vita J, Carvajal G, Egido J: Renal and vascular hypertension-induced inflammation: role of angiotensin II. Curr Opin Nephrol Hypertens. 2006 Mar;15(2):159-66. | | 5. Saavedra JM: Brain angiotensin II: new developments, unanswered questions and therapeutic opportunities. Cell Mol Neurobiol. 2005 Jun;25(3-4):485-512. | | 6. https://www.ncbi.nlm.nih.gov/pubmed/?term=16672146 |
|
|---|