| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:12:20 UTC |
|---|
| Update Date | 2016-11-09 01:17:22 UTC |
|---|
| Accession Number | CHEM021780 |
|---|
| Identification |
|---|
| Common Name | N-Ribosylhistidine |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | - FooDB Chemicals
- HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
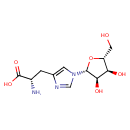
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| His-R | HMDB, MeSH | | N(Tau)-ribosylhistidine | HMDB, MeSH | | (2S)-2-Amino-3-{1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1H-imidazol-4-yl}propanoate | Generator, HMDB |
|
|---|
| Chemical Formula | C11H17N3O6 |
|---|
| Average Molecular Mass | 287.269 g/mol |
|---|
| Monoisotopic Mass | 287.112 g/mol |
|---|
| CAS Registry Number | 98379-91-0 |
|---|
| IUPAC Name | (2S)-2-amino-3-{1-[(2R,3R,4S,5R)-3,4-dihydroxy-5-(hydroxymethyl)oxolan-2-yl]-1H-imidazol-4-yl}propanoic acid |
|---|
| Traditional Name | his-R |
|---|
| SMILES | N[C@@H](CC1=CN(C=N1)[C@@H]1O[C@H](CO)[C@@H](O)[C@H]1O)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C11H17N3O6/c12-6(11(18)19)1-5-2-14(4-13-5)10-9(17)8(16)7(3-15)20-10/h2,4,6-10,15-17H,1,3,12H2,(H,18,19)/t6-,7+,8+,9+,10+/m0/s1 |
|---|
| InChI Key | TTYCFBAOLXCFAB-VAPHQMJDSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as histidine and derivatives. Histidine and derivatives are compounds containing cysteine or a derivative thereof resulting from reaction of cysteine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Histidine and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Histidine or derivatives
- Imidazole ribonucleoside
- Glycosyl compound
- N-glycosyl compound
- Alpha-amino acid
- Pentose monosaccharide
- L-alpha-amino acid
- Imidazolyl carboxylic acid derivative
- Aralkylamine
- Monosaccharide
- N-substituted imidazole
- Azole
- Heteroaromatic compound
- Imidazole
- Tetrahydrofuran
- Amino acid
- Secondary alcohol
- Carboxylic acid
- Oxacycle
- Azacycle
- Monocarboxylic acid or derivatives
- Organoheterocyclic compound
- Primary amine
- Organic oxide
- Primary aliphatic amine
- Organonitrogen compound
- Organooxygen compound
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Alcohol
- Carbonyl group
- Amine
- Hydrocarbon derivative
- Primary alcohol
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0c03-9340000000-73a8b2e9cc938d75e44d | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-03di-5931480000-4cb1f922181f0911e2b4 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4r-0970000000-759c0a607b1032d25416 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0bt9-0900000000-41b17087fc81992d35db | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-2900000000-f9d545c4e96bd470c45b | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-1590000000-f70782e731781bde63ea | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-2920000000-13d9d67f4af488782478 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0uki-8900000000-1be5db599106844bf7a9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0970000000-fc274386b5c899448b97 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ly9-3910000000-46e62b12bfe2a748410f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0cdi-7900000000-23583c1237b0c9d3c3d7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052r-0940000000-e3080474d7b607493432 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01p9-0900000000-e2042ee8b69a08b06af3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03e9-7910000000-5eab11cb72a4d406d4ff | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0002089 |
|---|
| FooDB ID | FDB022840 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | 6481 |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 112711 |
|---|
| ChEBI ID | 90055 |
|---|
| PubChem Compound ID | 126923 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|