| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:12:09 UTC |
|---|
| Update Date | 2016-11-09 01:17:21 UTC |
|---|
| Accession Number | CHEM021773 |
|---|
| Identification |
|---|
| Common Name | N2-Methylguanine |
|---|
| Class | Small Molecule |
|---|
| Description | A methylguanine in which the methyl group is located at the N2-position. |
|---|
| Contaminant Sources | - FooDB Chemicals
- HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
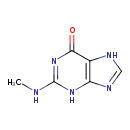
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 1,7-Dihydro-2-(methylamino)-6H-purin-6-one | ChEBI | | 2-Methylamino-6-oxopurine | ChEBI | | 2-Methylguanine | ChEBI | | N-Methyl-guanine | ChEBI | | 1, 7-dihydro-2-(methylamino)-6H-Purin-6-one | HMDB | | 2-(methylamino)-9H-Purin-6-ol | HMDB | | 2-methylamino-1,9-dihydro-Purin-6-one | HMDB | | 2-monomethylamino-6-Hydroxypurine | HMDB | | 6-Hydroxy-2-methylamino-purine | HMDB | | 6-Hydroxy-2-methylaminopurine | HMDB | | 7-Methylguanine | MeSH, HMDB | | N7-Me-g | MeSH, HMDB | | 7-Methylguanine, 14C-labeled | MeSH, HMDB | | N(2)-Methylguanine | MeSH, HMDB | | N7-Methylguanine | MeSH, HMDB | | N2-Methylguanine | MeSH |
|
|---|
| Chemical Formula | C6H7N5O |
|---|
| Average Molecular Mass | 165.153 g/mol |
|---|
| Monoisotopic Mass | 165.065 g/mol |
|---|
| CAS Registry Number | 10030-78-1 |
|---|
| IUPAC Name | 2-(methylamino)-6,7-dihydro-3H-purin-6-one |
|---|
| Traditional Name | 2-(methylamino)-3,7-dihydropurin-6-one |
|---|
| SMILES | CNC1=NC(=O)C2=C(N1)N=CN2 |
|---|
| InChI Identifier | InChI=1S/C6H7N5O/c1-7-6-10-4-3(5(12)11-6)8-2-9-4/h2H,1H3,(H3,7,8,9,10,11,12) |
|---|
| InChI Key | SGSSKEDGVONRGC-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as hypoxanthines. Hypoxanthines are compounds containing the purine derivative 1H-purin-6(9H)-one. Purine is a bicyclic aromatic compound made up of a pyrimidine ring fused to an imidazole ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Imidazopyrimidines |
|---|
| Sub Class | Purines and purine derivatives |
|---|
| Direct Parent | Hypoxanthines |
|---|
| Alternative Parents | |
|---|
| Substituents | - 6-oxopurine
- Hypoxanthine
- Aminopyrimidine
- Pyrimidone
- Secondary aliphatic/aromatic amine
- Pyrimidine
- Heteroaromatic compound
- Vinylogous amide
- Imidazole
- Azole
- Azacycle
- Secondary amine
- Organic nitrogen compound
- Amine
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00li-2900000000-539ed57cd0169be157e1 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0900000000-c5b4249fffca3d4d371f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-0900000000-e0e61dd036c1eeb8c7cd | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000b-9700000000-bc35e49ab2b1f81d19f2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0900000000-18f6ed24aee9629d2b71 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03dr-1900000000-3647b625be48894ded97 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00kf-9200000000-eb014bf17054735890c3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0900000000-11e5406fb2e0acd600c0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03el-1900000000-3dab99342f0796ffff0a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-568aee5531b232c43016 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-0900000000-7f20cf9333bbb1eb98e1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-1900000000-2641d6f2b8a568415740 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-9300000000-58b99541aa08fb42a838 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0006040 |
|---|
| FooDB ID | FDB023816 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 23213 |
|---|
| ChEBI ID | 21818 |
|---|
| PubChem Compound ID | 24828 |
|---|
| Kegg Compound ID | C04153 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. https://www.ncbi.nlm.nih.gov/pubmed/?term=17189261 | | 2. https://www.ncbi.nlm.nih.gov/pubmed/?term=19749381 | | 3. https://www.ncbi.nlm.nih.gov/pubmed/?term=23773213 | | 4. https://www.ncbi.nlm.nih.gov/pubmed/?term=24733044 | | 5. https://www.ncbi.nlm.nih.gov/pubmed/?term=4853458 | | 6. https://www.ncbi.nlm.nih.gov/pubmed/?term=5085644 | | 7. https://www.ncbi.nlm.nih.gov/pubmed/?term=7528943 | | 8. Choi JY, Guengerich FP: Kinetic evidence for inefficient and error-prone bypass across bulky N2-guanine DNA adducts by human DNA polymerase iota. J Biol Chem. 2006 May 5;281(18):12315-24. Epub 2006 Mar 8. | | 9. Choi JY, Guengerich FP: Adduct size limits efficient and error-free bypass across bulky N2-guanine DNA lesions by human DNA polymerase eta. J Mol Biol. 2005 Sep 9;352(1):72-90. | | 10. Porcelli B, Pagani R, Lorenzini L, De Martino A, Catinella S, Traldi P: Different mass spectrometric approaches in the identification of endogenous methylated purine bases in urine extracts. Rapid Commun Mass Spectrom. 1994 Jun;8(6):443-50. | | 11. Sander G, Topp H, Heller-Schoch G, Wieland J, Schoch G: Ribonucleic acid turnover in man:RNA catabolites in urine as measure for the metabolism of each of the three major species of RNA. Clin Sci (Lond). 1986 Oct;71(4):367-74. | | 12. Kanduc D, Sapia G: Origin of 1,7-dimethylguanosine in tRNA chemical and enzymatic methylation. Boll Soc Ital Biol Sper. 1982 Oct 15;58(19):1221-5. | | 13. Niwa T, Takeda N, Yoshizumi H: RNA metabolism in uremic patients: accumulation of modified ribonucleosides in uremic serum. Technical note. Kidney Int. 1998 Jun;53(6):1801-6. |
|
|---|