| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:11:45 UTC |
|---|
| Update Date | 2016-11-09 01:17:21 UTC |
|---|
| Accession Number | CHEM021758 |
|---|
| Identification |
|---|
| Common Name | A,b-Dihydroxyisobutyric acid |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | - FooDB Chemicals
- HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
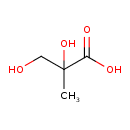
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2,3-Dihydroxy-2-methylpropionic acid | ChEBI | | alpha,beta-Dihydroxyisobutyric acid | ChEBI | | 2,3-Dihydroxy-2-methylpropionate | Generator | | a,b-Dihydroxyisobutyrate | Generator | | alpha,beta-Dihydroxyisobutyrate | Generator | | Α,β-dihydroxyisobutyrate | Generator | | Α,β-dihydroxyisobutyric acid | Generator | | 2,3-Dihydroxy-2-methyl-propanoate | HMDB | | 2,3-Dihydroxy-2-methyl-propanoic acid | HMDB | | 2,3-Dihydroxy-2-methylpropanoate | HMDB | | 2,3-Dihydroxy-2-methylpropanoic acid | HMDB | | 2-C-Methylglycerate | HMDB | | 2-C-Methylglyceric acid | HMDB | | 2-Methyl-2,3-dihydroxypropionate | HMDB | | 2-Methyl-2,3-dihydroxypropionic acid | HMDB | | 2-Methylglycerate | HMDB | | 2-Methylglyceric acid | HMDB | | 2-Methylglyceronate | HMDB | | 2-Methylglyceronic acid | HMDB | | a-Methylglycerate | HMDB | | a-Methylglyceric acid | HMDB | | alpha-Methylglycerate | HMDB | | alpha-Methylglyceric acid | HMDB | | a,b-Dihydroxyisobutyric acid | Generator | | a,b-Dihydroxy-isobutyrate | Generator, HMDB |
|
|---|
| Chemical Formula | C4H8O4 |
|---|
| Average Molecular Mass | 120.104 g/mol |
|---|
| Monoisotopic Mass | 120.042 g/mol |
|---|
| CAS Registry Number | 21620-60-0 |
|---|
| IUPAC Name | 2,3-dihydroxy-2-methylpropanoic acid |
|---|
| Traditional Name | 2-methylglyceric acid |
|---|
| SMILES | CC(O)(CO)C(O)=O |
|---|
| InChI Identifier | InChI=1S/C4H8O4/c1-4(8,2-5)3(6)7/h5,8H,2H2,1H3,(H,6,7) |
|---|
| InChI Key | DGADNPLBVRLJGD-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as beta hydroxy acids and derivatives. Beta hydroxy acids and derivatives are compounds containing a carboxylic acid substituted with a hydroxyl group on the C3 carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Hydroxy acids and derivatives |
|---|
| Sub Class | Beta hydroxy acids and derivatives |
|---|
| Direct Parent | Beta hydroxy acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Beta-hydroxy acid
- Alpha-hydroxy acid
- Tertiary alcohol
- 1,2-diol
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9000000000-e1621256a3593b108d7b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-00xr-9483000000-65c48fae8da1c633d6e0 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-5900000000-1e5b5bdae0747362e72e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0kdi-9300000000-095b57159a1bf51f99e6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-9000000000-c11a68b5d083815170fb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-5900000000-8d727db5f35d4b5b4f5a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-009i-9100000000-ba0f4e2e0107afb4a8eb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9000000000-507242d2271f378a8b48 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0gb9-4900000000-c5f0ac9727f0a3efdfc0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00dr-9100000000-3787459d4219bd7ca7f3 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9000000000-d86f752a19333583745e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0zfr-9800000000-2220e44bfb31db07608e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-9000000000-86f9f5a80900ccc1a671 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052e-9000000000-d1253ed770e4b16d8e79 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0002601 |
|---|
| FooDB ID | FDB023033 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | 6723 |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 487503 |
|---|
| ChEBI ID | 36532 |
|---|
| PubChem Compound ID | 560781 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|