| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:10:32 UTC |
|---|
| Update Date | 2016-11-09 01:17:20 UTC |
|---|
| Accession Number | CHEM021708 |
|---|
| Identification |
|---|
| Common Name | Epinephrine sulfate |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | - FooDB Chemicals
- HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
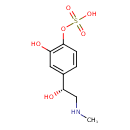
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| Epinephrine sulfuric acid | Generator | | Epinephrine sulphate | Generator | | Epinephrine sulphuric acid | Generator | | Epinephrine sulfoconjugate | MeSH | | Epinephrine-3-O-sulfate | MeSH | | (R)-4-[1-Hydroxy-2-(methylamino)ethyl]-2-benzenediol mono(hydrogen sulfate) (ester) | HMDB | | (R)-4-[1-Hydroxy-2-(methylamino)ethyl]-2-benzenediol mono(hydrogen sulphate) (ester) | HMDB | | Adrenaline sulfate | HMDB | | Adrenaline sulphate | HMDB | | {2-hydroxy-4-[(1R)-1-hydroxy-2-(methylamino)ethyl]phenyl}oxidanesulfonate | Generator, HMDB | | {2-hydroxy-4-[(1R)-1-hydroxy-2-(methylamino)ethyl]phenyl}oxidanesulphonate | Generator, HMDB | | {2-hydroxy-4-[(1R)-1-hydroxy-2-(methylamino)ethyl]phenyl}oxidanesulphonic acid | Generator, HMDB | | Epinephrine sulfate | MeSH |
|
|---|
| Chemical Formula | C9H13NO6S |
|---|
| Average Molecular Mass | 263.268 g/mol |
|---|
| Monoisotopic Mass | 263.046 g/mol |
|---|
| CAS Registry Number | 77469-50-2 |
|---|
| IUPAC Name | {2-hydroxy-4-[(1R)-1-hydroxy-2-(methylamino)ethyl]phenyl}oxidanesulfonic acid |
|---|
| Traditional Name | epinephrine sulfoconjugate |
|---|
| SMILES | CNC[C@H](O)C1=CC(O)=C(OS(O)(=O)=O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C9H13NO6S/c1-10-5-8(12)6-2-3-9(7(11)4-6)16-17(13,14)15/h2-4,8,10-12H,5H2,1H3,(H,13,14,15)/t8-/m0/s1 |
|---|
| InChI Key | AELFRHHZGTVYGJ-QMMMGPOBSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylsulfates. Phenylsulfates are compounds containing a sulfuric acid group conjugated to a phenyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Organic sulfuric acids and derivatives |
|---|
| Sub Class | Arylsulfates |
|---|
| Direct Parent | Phenylsulfates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenylsulfate
- Phenoxy compound
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Aralkylamine
- Monocyclic benzene moiety
- Benzenoid
- Sulfuric acid ester
- Sulfate-ester
- Sulfuric acid monoester
- 1,2-aminoalcohol
- Secondary alcohol
- Secondary aliphatic amine
- Secondary amine
- Organic nitrogen compound
- Aromatic alcohol
- Hydrocarbon derivative
- Organic oxide
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Amine
- Alcohol
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9220000000-2ece4d03b2567c81287b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-01bc-9647000000-df7d020cb12314aed975 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-01ot-0090000000-10698a49856be4dc14e9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014j-1690000000-404ceebd15110a71f4db | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f7p-9530000000-2681df63164cd1374dd4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-1090000000-c14175b75803b9f650fc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03e9-2940000000-cc1a9d9f83dd8f4a2240 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0kar-5900000000-42cb1da8290c53d480af | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-03di-0090000000-1726b56c3cfad8e33ad9 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-0090000000-31c25ad902fcf76c14d5 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0002-9010000000-df1a8f8ec88eb7dc517f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kb-0390000000-893a0d25871ffe945f6c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-02t9-0950000000-7e6cb13e33b943cc78b1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-2900000000-99b26eca74d6f3adb02a | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0001876 |
|---|
| FooDB ID | FDB022721 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 2299690 |
|---|
| ChEBI ID | 89878 |
|---|
| PubChem Compound ID | 3035453 |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|