| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:10:30 UTC |
|---|
| Update Date | 2016-11-09 01:17:20 UTC |
|---|
| Accession Number | CHEM021707 |
|---|
| Identification |
|---|
| Common Name | Nicotinamide ribotide |
|---|
| Class | Small Molecule |
|---|
| Description | Nicotinamide ribotide (NMN) is an important intermediate metabolite in the nicotinate and nicotinamide metabolism pathway. Mammals predominantly use nicotinamide rather than nicotinic acid as a precursor for NAD biosynthesis. Instead of the deamidation to nicotinic acid, nicotinamide is directly converted to Nicotinamide ribotide by nicotinamide phosphoribosyltransferase (NAMPT, EC 2.4.2.12). The enzyme Nicotinamide ribotide adenylyltransferase (NMNAT, EC 2.7.7.1), a member of the nucleotidyltransferase alpha/beta-phosphodiesterase superfamily, catalyzes the reaction Nicotinamide ribotide + ATP = Nicotinamide adenine dinucleotide (NAD) + PPi, representing the final step in the biosynthesis of NAD, a molecule playing a fundamental role as a cofactor in cellular redox reactions. Thus Nicotinamide ribotide is an important metabolite for the maintenance of normal NAD biosynthesis, and circulating Nicotinamide ribotide levels may play an important role in regulating cell function in physiological and pathophysiological conditions. (PMID: 15078171, 17983582) |
|---|
| Contaminant Sources | - FooDB Chemicals
- HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
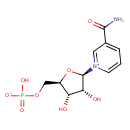
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 3-(Aminocarbonyl)-1-(5-O-phosphonato-beta-D-ribofuranosyl)pyridinium | ChEBI | | 3-(Aminocarbonyl)-1-(5-O-phosphono-beta-D-ribofuranosyl)pyridinium, inner salt | ChEBI | | beta-Nicotinamide D-ribonucleotide | ChEBI | | beta-Nicotinamide mononucleotide | ChEBI | | beta-Nicotinamide ribonucleotide | ChEBI | | Nicotinamide D-ribonucleotide | ChEBI | | Nicotinamide mononucleotide | ChEBI | | Nicotinamide nucleotide | ChEBI | | Nicotinamide ribonucleotide | ChEBI | | NMN | ChEBI | | 3-(Aminocarbonyl)-1-(5-O-phosphonato-b-D-ribofuranosyl)pyridinium | Generator | | 3-(Aminocarbonyl)-1-(5-O-phosphonato-β-D-ribofuranosyl)pyridinium | Generator | | 3-(Aminocarbonyl)-1-(5-O-phosphono-b-D-ribofuranosyl)pyridinium, inner salt | Generator | | 3-(Aminocarbonyl)-1-(5-O-phosphono-β-D-ribofuranosyl)pyridinium, inner salt | Generator | | b-Nicotinamide D-ribonucleotide | Generator | | Β-nicotinamide D-ribonucleotide | Generator | | b-Nicotinamide mononucleotide | Generator | | Β-nicotinamide mononucleotide | Generator | | b-Nicotinamide ribonucleotide | Generator | | Β-nicotinamide ribonucleotide | Generator | | Mononucleotide, nicotinamide | MeSH | | 3-(Aminocarbonyl)-1-(5-O-phosphono-b-D-ribofuranosyl)-pyridinium hydroxide inner salt | HMDB | | 3-(Aminocarbonyl)-1-(5-O-phosphono-b-D-ribofuranosyl)-pyridinium inner salt | HMDB | | 3-(Aminocarbonyl)-1-(5-O-phosphono-beta-delta-ribofuranosyl)-pyridinium hydroxide inner salt | HMDB | | 3-(Aminocarbonyl)-1-(5-O-phosphono-beta-delta-ribofuranosyl)-pyridinium inner salt | HMDB | | 3-Carbamoyl-1-b-D-ribofuranosylpyridinium hydroxide 5'-phosphate inner salt | HMDB | | 3-Carbamoyl-1-beta-delta-ribofuranosylpyridinium hydroxide 5'-phosphate inner salt | HMDB | | b-D-NMN | HMDB | | b-NMN | HMDB | | beta-delta-NMN | HMDB | | beta-NMN | HMDB | | Nicotinamide ribonucleoside 5'-phosphate | HMDB | | Bibenzoyl | HMDB | | Diphenylethanedione | HMDB | | Diphenylglyoxal | HMDB | | 1,2-Diphenylethane-1,2-dione | HMDB | | Diphenylethane-1,2-dione | HMDB | | Diphenyl-alpha-beta-ketone | HMDB | | Phosphatidylinositol 4,5-bisphosphoric acid | HMDB | | Diadenosine triphosphoric acid | HMDB | | Adenosine (5')triphospho(5')adenosine | HMDB | | Adenosine 5'-triphosphate 5'-adenosine | HMDB | | Adenosine(3)triphosphate adenosine | HMDB | | Adenosine(5')triphospho(5')adenosine | HMDB | | Bis(adenosine)-5'-triphosphate | HMDB | | p(1),p(3)-Bis(5'-adenosyl) trihydrogen triphosphate | HMDB | | p(1),p(3)-Bis(5'-adenosyl) triphosphate | HMDB | | p(1)-p(3)-Bis(5'-adenosyl) triphosphate | HMDB | | P1,P3-Bis(5'-adenosyl) triphosphate | HMDB | | Ap3a | HMDB | | 5'Ap3a | HMDB | | p(1),(P3)-Bis(5'-adenosyl)triphosphate | HMDB | | ApppA | HMDB | | Tetrahydrofolate | HMDB | | (6S)-Tetrahydrofolate | HMDB | | (6S)-Tetrahydrofolic acid | HMDB | | 5,6,7,8-Tetrahydrofolate | HMDB | | 5,6,7,8-Tetrahydrofolic acid | HMDB | | Tetra-H-folate | HMDB | | Tetrahydrafolate | HMDB | | Tetrahydropteroyl mono-L-glutamate | HMDB | | Tetrahydropteroylglutamate | HMDB | | 1,4-Butanediamine | HMDB | | 1,4-Butylenediamine | HMDB | | 1,4-DIAMINOBUTANE | HMDB | | 1,4-Tetramethylenediamine | HMDB | | Butane-1,4-diamine | HMDB | | Butylenediamine | HMDB | | H2N(CH2)4nh2 | HMDB | | Putrescin | HMDB | | Putrescina | HMDB | | Putreszin | HMDB | | Tetramethylendiamin | HMDB | | Tetramethylenediamine | HMDB | | 1,4-Butanediammonium | HMDB | | Tetramethyldiamine | HMDB | | 1,4 Diaminobutane | HMDB | | 1,4 Butanediamine | HMDB |
|
|---|
| Chemical Formula | C11H15N2O8P |
|---|
| Average Molecular Mass | 334.219 g/mol |
|---|
| Monoisotopic Mass | 334.057 g/mol |
|---|
| CAS Registry Number | 1094-61-7 |
|---|
| IUPAC Name | 3-carbamoyl-1-[(2R,3R,4S,5R)-5-[(hydrogen phosphonooxy)methyl]-3,4-dihydroxyoxolan-2-yl]-1lambda5-pyridin-1-ylium |
|---|
| Traditional Name | 3-carbamoyl-1-[(2R,3R,4S,5R)-5-[(hydrogen phosphonooxy)methyl]-3,4-dihydroxyoxolan-2-yl]-1lambda5-pyridin-1-ylium |
|---|
| SMILES | NC(=O)C1=CC=C[N+](=C1)[C@@H]1O[C@H](COP(O)([O-])=O)[C@@H](O)[C@H]1O |
|---|
| InChI Identifier | InChI=1S/C11H15N2O8P/c12-10(16)6-2-1-3-13(4-6)11-9(15)8(14)7(21-11)5-20-22(17,18)19/h1-4,7-9,11,14-15H,5H2,(H3-,12,16,17,18,19)/t7-,8-,9-,11-/m1/s1 |
|---|
| InChI Key | DAYLJWODMCOQEW-TURQNECASA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as nicotinamide nucleotides. These are pyridine nucleotides, in which the pyridine base is nicotinamide or a derivative thereof. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
| Class | Pyridine nucleotides |
|---|
| Sub Class | Nicotinamide nucleotides |
|---|
| Direct Parent | Nicotinamide nucleotides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Nicotinamide-nucleotide
- Pentose-5-phosphate
- Pentose phosphate
- Glycosyl compound
- N-glycosyl compound
- Pentose monosaccharide
- Monosaccharide phosphate
- Nicotinamide
- Pyridine carboxylic acid or derivatives
- Monosaccharide
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Pyridinium
- Pyridine
- Heteroaromatic compound
- Vinylogous amide
- Tetrahydrofuran
- 1,2-diol
- Carboxamide group
- Secondary alcohol
- Primary carboxylic acid amide
- Azacycle
- Oxacycle
- Carboxylic acid derivative
- Organoheterocyclic compound
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Organic zwitterion
- Alcohol
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-006t-8902000000-75d46c23151f03298e05 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-01b9-4920300000-f0eec1327b9e3e217fdd | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-00di-1931000000-5aa0e5ba467b6bdbb57f | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-00dj-6920000000-4720b1b8ab108dc1b499 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-00mk-8903000000-042bd96d57e63adafe44 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF (UPLC Q-Tof Premier, Waters) , Positive | splash10-00di-3900000000-013c2f02e48342e61b5e | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-00di-3900000000-013c2f02e48342e61b5e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-1109000000-7e48df4297f6933ffc64 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-002b-7419000000-c70e04daa2249a30318c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0005-9600000000-ec07884c9a438ebdba5a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-1009000000-623a45d318f08c622565 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001i-9002000000-a9b586e58b5858c6d213 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0032-9000000000-3fde5442f77c9f4b54b3 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 2D NMR | [1H,1H] 2D NMR Spectrum | Not Available | Spectrum | | 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0000229 |
|---|
| FooDB ID | FDB021912 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00019694 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | 16171 |
|---|
| PubChem Compound ID | 14180 |
|---|
| Kegg Compound ID | C00455 |
|---|
| YMDB ID | YMDB00138 |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|