| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:10:25 UTC |
|---|
| Update Date | 2016-11-09 01:17:20 UTC |
|---|
| Accession Number | CHEM021702 |
|---|
| Identification |
|---|
| Common Name | Dopamine 3-O-sulfate |
|---|
| Class | Small Molecule |
|---|
| Description | An aryl sulfate that is dopamine in which the phenolic hydrogen at position 3 has been replaced by a sulfo group. |
|---|
| Contaminant Sources | - FooDB Chemicals
- HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
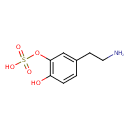
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 4-(2-Aminoethyl)-1,2-benzenediol 2-(hydrogen sulfate) | ChEBI | | [5-(2-Aminoethyl)-2-hydroxyphenyl]oxidanesulfonic acid | ChEBI | | Dopamine 3-monosulfate | ChEBI | | Dopamine 3-sulfate | ChEBI | | 4-(2-Aminoethyl)-1,2-benzenediol 2-(hydrogen sulfuric acid) | Generator | | 4-(2-Aminoethyl)-1,2-benzenediol 2-(hydrogen sulphate) | Generator | | 4-(2-Aminoethyl)-1,2-benzenediol 2-(hydrogen sulphuric acid) | Generator | | [5-(2-Aminoethyl)-2-hydroxyphenyl]oxidanesulfonate | Generator | | [5-(2-Aminoethyl)-2-hydroxyphenyl]oxidanesulphonate | Generator | | [5-(2-Aminoethyl)-2-hydroxyphenyl]oxidanesulphonic acid | Generator | | Dopamine 3-monosulfuric acid | Generator | | Dopamine 3-monosulphate | Generator | | Dopamine 3-monosulphuric acid | Generator | | Dopamine 3-sulfuric acid | Generator | | Dopamine 3-sulphate | Generator | | Dopamine 3-sulphuric acid | Generator | | Dopamine 3-O-sulfuric acid | Generator | | Dopamine 3-O-sulphate | Generator | | Dopamine 3-O-sulphuric acid | Generator |
|
|---|
| Chemical Formula | C8H11NO5S |
|---|
| Average Molecular Mass | 233.242 g/mol |
|---|
| Monoisotopic Mass | 233.036 g/mol |
|---|
| CAS Registry Number | 51317-41-0 |
|---|
| IUPAC Name | [5-(2-aminoethyl)-2-hydroxyphenyl]oxidanesulfonic acid |
|---|
| Traditional Name | dopamine 3-O-sulfate |
|---|
| SMILES | NCCC1=CC(OS(O)(=O)=O)=C(O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C8H11NO5S/c9-4-3-6-1-2-7(10)8(5-6)14-15(11,12)13/h1-2,5,10H,3-4,9H2,(H,11,12,13) |
|---|
| InChI Key | NZKRYJGNYPYXJZ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylsulfates. Phenylsulfates are compounds containing a sulfuric acid group conjugated to a phenyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Organic sulfuric acids and derivatives |
|---|
| Sub Class | Arylsulfates |
|---|
| Direct Parent | Phenylsulfates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenylsulfate
- Phenethylamine
- Phenoxy compound
- 2-arylethylamine
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Aralkylamine
- Monocyclic benzene moiety
- Sulfuric acid monoester
- Sulfate-ester
- Sulfuric acid ester
- Benzenoid
- Primary amine
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Organooxygen compound
- Amine
- Primary aliphatic amine
- Organonitrogen compound
- Organic oxide
- Hydrocarbon derivative
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001i-9650000000-2db72db7d63697e55bd1 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0fl0-9320000000-ace4fcc9cdb2373f726c | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0159-0090000000-b2228f1210694d4037be | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014r-1950000000-c9ce0c682709c1c90e1d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gdi-9300000000-f413b35bca5b45692bb0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0090000000-c18c16811fdd8fb7e8d7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-1930000000-37551dd11cac8f51977a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0f8i-5900000000-6729c4999d2afb12eec0 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-0090000000-b5c60a607f4b8ffe8309 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001j-5090000000-6dc0ebdf64e66d55361a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-01ow-9100000000-c48fe767b4f0b00bc40e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0920000000-5bc862fdad49e730b60a | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0900000000-751f9057ce9f08d81036 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-066u-5900000000-9d59879b17c28b88d464 | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0006275 |
|---|
| FooDB ID | FDB023872 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | 2320809 |
|---|
| BioCyc ID | CPD-7649 |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Not Available |
|---|
| Chemspider ID | 108936 |
|---|
| ChEBI ID | 37946 |
|---|
| PubChem Compound ID | 122136 |
|---|
| Kegg Compound ID | C13690 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. https://www.ncbi.nlm.nih.gov/pubmed/?term=1195131 | | 2. https://www.ncbi.nlm.nih.gov/pubmed/?term=17548063 | | 3. https://www.ncbi.nlm.nih.gov/pubmed/?term=4055948 | | 4. https://www.ncbi.nlm.nih.gov/pubmed/?term=571950 | | 5. https://www.ncbi.nlm.nih.gov/pubmed/?term=6496664 | | 6. https://www.ncbi.nlm.nih.gov/pubmed/?term=938583 | | 7. Osikowska, Barbara A.; Idle, J. R.; Swinbourne, F. J.; Sever, P. S. Unequivocal synthesis and characterization of dopamine 3- and 4-O-sulfates. Biochemical Pharmacology (1982), 31(13), 2279-84. | | 8. Osikowska, Barbara A.; Idle, J. R.; Swinbourne, F. J.; Sever, P. S. Unequivocal synthesis and characterization of dopamine 3- and 4-O-sulfates. Biochemical Pharmacology (1982), 31(13), 2279-84. | | 9. Itaaho K, Alakurtti S, Yli-Kauhaluoma J, Taskinen J, Coughtrie MW, Kostiainen R: Regioselective sulfonation of dopamine by SULT1A3 in vitro provides a molecular explanation for the preponderance of dopamine-3-O-sulfate in human blood circulation. Biochem Pharmacol. 2007 Aug 1;74(3):504-10. Epub 2007 May 10. |
|
|---|