| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:09:51 UTC |
|---|
| Update Date | 2016-11-09 01:17:20 UTC |
|---|
| Accession Number | CHEM021680 |
|---|
| Identification |
|---|
| Common Name | Metanephrine |
|---|
| Class | Small Molecule |
|---|
| Description | Metanephrine, also known as metanephrine, belongs to the class of organic compounds known as methoxyphenols. Methoxyphenols are compounds containing a methoxy group attached to the benzene ring of a phenol moiety. Metanephrine is possibly soluble (in water) and a very strong basic compound (based on its pKa). Metanephrine participates in a number of enzymatic reactions, within cattle. In particular, Metanephrine can be converted into 3-methoxy-4-hydroxyphenylglycolaldehyde; which is mediated by the enzyme amine oxidase [flavin-containing] a. In addition, Metanephrine and pyrocatechol can be biosynthesized from epinephrine and guaiacol; which is mediated by the enzyme catechol O-methyltransferase. In cattle, metanephrine is involved in the metabolic pathway called the tyrosine metabolism pathway. |
|---|
| Contaminant Sources | - FooDB Chemicals
- HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
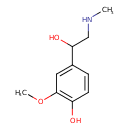
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| (+/-)-metanephrine | HMDB | | 3-Methoxy-adrenaline | HMDB | | 3-Methoxyadrenaline | HMDB | | 3-O-Methyl-adrenaline | HMDB | | 3-O-Methylepinephrine | HMDB | | 4-Hydroxy-3-methoxy-N-methylphenethanolamine | HMDB | | DL-3-O-Methyladrenaline | HMDB | | DL-Metanephrine | HMDB | | m-O-Methyladrenaline | HMDB | | Metadrenaline | HMDB |
|
|---|
| Chemical Formula | C10H15NO3 |
|---|
| Average Molecular Mass | 197.231 g/mol |
|---|
| Monoisotopic Mass | 197.105 g/mol |
|---|
| CAS Registry Number | 5001-33-2 |
|---|
| IUPAC Name | 4-[1-hydroxy-2-(methylamino)ethyl]-2-methoxyphenol |
|---|
| Traditional Name | (+/-)-metanephrine |
|---|
| SMILES | CNCC(O)C1=CC(OC)=C(O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C10H15NO3/c1-11-6-9(13)7-3-4-8(12)10(5-7)14-2/h3-5,9,11-13H,6H2,1-2H3 |
|---|
| InChI Key | JWJCTZKFYGDABJ-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as methoxyphenols. Methoxyphenols are compounds containing a methoxy group attached to the benzene ring of a phenol moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Phenols |
|---|
| Sub Class | Methoxyphenols |
|---|
| Direct Parent | Methoxyphenols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Methoxyphenol
- Phenoxy compound
- Anisole
- Phenol ether
- Methoxybenzene
- Alkyl aryl ether
- 1-hydroxy-2-unsubstituted benzenoid
- Aralkylamine
- Monocyclic benzene moiety
- Secondary alcohol
- 1,2-aminoalcohol
- Secondary amine
- Ether
- Secondary aliphatic amine
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic oxygen compound
- Amine
- Organic nitrogen compound
- Alcohol
- Aromatic alcohol
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (3 TMS) | splash10-014i-0910000000-a886bef599ae583766b9 | Spectrum | | GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-014i-0910000000-a886bef599ae583766b9 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9300000000-99e319cf43defe7cc611 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0g6r-9553000000-f11ff40fb7a8af16bb25 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001j-0900000000-4c31604c1b2a54419f92 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001j-0900000000-12556e41dd73a4575162 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f6w-4900000000-403de00b0b43ed22918f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-3d29615883725ba8e07c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0032-1900000000-1ccaa0d8ce9764cdfe35 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0pic-4900000000-051892c7895245a3de0c | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001j-0900000000-545ae65ccf6b1aaf8f43 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-001a-1900000000-ad33b1d4593df03f60e1 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-5900000000-61cd5865cf7abd2bba67 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-33b506e73b8f2abe6bdb | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-000b-0900000000-361e5b74103dd493ac47 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4m-6900000000-49bdcf483f24e0b4ffd4 | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum | | 1D NMR | 13C NMR Spectrum | Not Available | Spectrum | | 1D NMR | 1H NMR Spectrum | Not Available | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0004063 |
|---|
| FooDB ID | FDB023296 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | 46078 |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | 65 |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Metanephrine |
|---|
| Chemspider ID | 19844 |
|---|
| ChEBI ID | 144365 |
|---|
| PubChem Compound ID | 21100 |
|---|
| Kegg Compound ID | C05588 |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | | 1. van Dam PS, van Gils A, Canninga-van Dijk MR, de Koning EJ, Hofland LJ, de Herder WW: Sequential ACTH and catecholamine secretion in a phaeochromocytoma. Eur J Endocrinol. 2002 Aug;147(2):201-6. | | 2. Schmidt J, Mohr VD, Metzger P, Zirngibl H: Posttraumatic hypertension secondary to adrenal hemorrhage mimicking pheochromocytoma: case report. J Trauma. 1999 May;46(5):973-5. | | 3. Artal R, Platt LD, Kammula RK, Strassner HT, Gratacos J, Golde SH: Sympathoadrenal activity in infants of diabetic mothers. Am J Obstet Gynecol. 1982 Feb 15;142(4):436-9. | | 4. Uchikura K, Horikawa R, Tanimura T, Kabasawa Y: Determination of catecholamines by radioenzymatic assay using ion-pair liquid chromatography. J Chromatogr. 1981 Apr 10;223(1):41-50. | | 5. Artal R, Hobel CJ, Lam R, Oddie TH, Fisher DA: Free metanephrine in human amniotic fluid as an index of fetal sympathetic nervous system maturation. Am J Obstet Gynecol. 1979 Feb 15;133(4):452-4. | | 6. Kitamura T, Alroy J, Gatmaitan Z, Inoue M, Mikami T, Jansen P, Arias IM: Defective biliary excretion of epinephrine metabolites in mutant (TR-) rats: relation to the pathogenesis of black liver in the Dubin-Johnson syndrome and Corriedale sheep with an analogous excretory defect. Hepatology. 1992 Jun;15(6):1154-9. | | 7. Eisenhofer G, Keiser H, Friberg P, Mezey E, Huynh TT, Hiremagalur B, Ellingson T, Duddempudi S, Eijsbouts A, Lenders JW: Plasma metanephrines are markers of pheochromocytoma produced by catechol-O-methyltransferase within tumors. J Clin Endocrinol Metab. 1998 Jun;83(6):2175-85. | | 8. Higa S, Suzuki T, Sakoda S, Kishimoto S, Takaba Y, Nakajima A, Markey SP: Disturbed function of the pineal gland in familial amyloid polyneuropathy. J Neural Transm. 1987;69(1-2):97-103. | | 9. Beck O, Faull KF: Extractive acylation and mass spectrometric assay of 3-methoxytyramine, normetanephrine, and metanephrine in cerebrospinal fluid. Anal Biochem. 1985 Sep;149(2):492-500. | | 10. Goldstein DS, Eisenhofer G, Kopin IJ: Sources and significance of plasma levels of catechols and their metabolites in humans. J Pharmacol Exp Ther. 2003 Jun;305(3):800-11. Epub 2003 Mar 20. | | 11. Pagliari R, Cottet-Emard JM, Peyrin L: Determination of free and conjugated normetanephrine and metanephrine in human plasma by high-performance liquid chromatography with electrochemical detection. J Chromatogr. 1991 Jan 18;563(1):23-36. | | 12. Kaplan NM, Kramer NJ, Holland OB, Sheps SG, Gomez-Sanchez C: Single-voided urine metanephrine assays in screening for pheochromocytoma. Arch Intern Med. 1977 Feb;137(2):190-3. | | 13. Taylor RL, Singh RJ: Validation of liquid chromatography-tandem mass spectrometry method for analysis of urinary conjugated metanephrine and normetanephrine for screening of pheochromocytoma. Clin Chem. 2002 Mar;48(3):533-9. | | 14. Eisenhofer G, Kopin IJ, Goldstein DS: Catecholamine metabolism: a contemporary view with implications for physiology and medicine. Pharmacol Rev. 2004 Sep;56(3):331-49. |
|
|---|