| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:09:05 UTC |
|---|
| Update Date | 2016-11-09 01:17:20 UTC |
|---|
| Accession Number | CHEM021647 |
|---|
| Identification |
|---|
| Common Name | Cyanidin |
|---|
| Class | Small Molecule |
|---|
| Description | 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-1λ⁴-chromen-1-ylium is a predicted metabolite generated by BioTransformer¹ that is produced by the metabolism of 2-(3,4-dihydroxyphenyl)-5,7-dihydroxy-3-{[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}-1λ⁴-chromen-1-ylium. It is generated by EC.3.2.1.X enzyme via a glycoside-hydrolysis reaction. This glycoside-hydrolysis occurs in human gut microbiota. |
|---|
| Contaminant Sources | - FooDB Chemicals
- HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
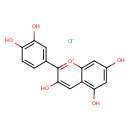
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 2-(3,4-Dihydroxyphenyl)-3,5,7-trihydroxy-1-benzopyrylium chloride | HMDB | | 3,3',4',5,7-Pentahydroxy-2-phenylbenzopyrylium chloride | HMDB | | 3,3',4',5,7-Pentahydroxyflavylium chloride | HMDB | | Cyanidin chloride | HMDB | | Cyanidine | HMDB | | Cyanidol | HMDB | | Cyanidol chloride | HMDB | | IdB 1027 | HMDB | | 2-(3,4-Dihydroxyphenyl) chromenylium-3,5,7-triol | MeSH | | 3,5,7,3',4'-Pentahydroxyflavylium | MeSH | | Cyanidin cation | MeSH | | 1-Benzopyrylium, 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-, chloride | MeSH | | Cyanidin ion | MeSH | | Flavylium, 3,3',4',5,7-pentahydroxy-, chloride | MeSH | | 1-Benzopyrylium, 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-, chloride (1:1) | MeSH | | Cyanidin | MeSH |
|
|---|
| Chemical Formula | C15H11ClO6 |
|---|
| Average Molecular Mass | 322.697 g/mol |
|---|
| Monoisotopic Mass | 322.024 g/mol |
|---|
| CAS Registry Number | 528-58-5 |
|---|
| IUPAC Name | 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-1lambda4-chromen-1-ylium chloride |
|---|
| Traditional Name | 2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-1lambda4-chromen-1-ylium chloride |
|---|
| SMILES | [Cl-].OC1=CC(O)=C2C=C(O)C(=[O+]C2=C1)C1=CC(O)=C(O)C=C1 |
|---|
| InChI Identifier | InChI=1S/C15H10O6.ClH/c16-8-4-11(18)9-6-13(20)15(21-14(9)5-8)7-1-2-10(17)12(19)3-7;/h1-6H,(H4-,16,17,18,19,20);1H |
|---|
| InChI Key | COAWNPJQKJEHPG-UHFFFAOYSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 7-hydroxyflavonoids. These are flavonoids that bear one hydroxyl group at the C-7 position of the flavonoid skeleton. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Flavonoids |
|---|
| Sub Class | Hydroxyflavonoids |
|---|
| Direct Parent | 7-hydroxyflavonoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - 3'-hydroxyflavonoid
- 3-hydroxyflavonoid
- 4'-hydroxyflavonoid
- 7-hydroxyflavonoid
- 5-hydroxyflavonoid
- Anthocyanidin
- Benzopyran
- 1-benzopyran
- Catechol
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Monocyclic benzene moiety
- Benzenoid
- Heteroaromatic compound
- Polyol
- Oxacycle
- Organoheterocyclic compound
- Organic oxygen compound
- Organooxygen compound
- Organic zwitterion
- Organic salt
- Organic chloride salt
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0ab9-0981000000-b03145f35dffd7b73034 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (5 TMS) - 70eV, Positive | splash10-001i-2062095000-78127582ff2f81e09993 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0009000000-f9880830ba79067d96b6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00di-0009000000-f9880830ba79067d96b6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00di-0009000000-f9880830ba79067d96b6 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0009000000-eee166fbbcec693fd35d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0009000000-eee166fbbcec693fd35d | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00di-0009000000-eee166fbbcec693fd35d | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | HMDB0125127 |
|---|
| FooDB ID | FDB002602 |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | C00006614 |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | CPD-7632 |
|---|
| METLIN ID | 3413 |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Cyanidin |
|---|
| Chemspider ID | 61546 |
|---|
| ChEBI ID | 604446 |
|---|
| PubChem Compound ID | 68247 |
|---|
| Kegg Compound ID | C05905 |
|---|
| YMDB ID | YMDB01659 |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | |
|---|