| Record Information |
|---|
| Version | 1.0 |
|---|
| Creation Date | 2016-05-25 18:09:01 UTC |
|---|
| Update Date | 2016-11-09 01:17:20 UTC |
|---|
| Accession Number | CHEM021644 |
|---|
| Identification |
|---|
| Common Name | Leukotriene E4 |
|---|
| Class | Small Molecule |
|---|
| Description | |
|---|
| Contaminant Sources | - FooDB Chemicals
- HMDB Contaminants - Urine
|
|---|
| Contaminant Type | Not Available |
|---|
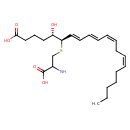
| Chemical Structure | |
|---|
| Synonyms | | Value | Source |
|---|
| 20-Hydroxy-leukotriene e4 | HMDB | | 20-OH-Leukotriene e4 | HMDB | | LTE4 | HMDB | | [5S-[5R*,6S*(s*),7E,9E,11Z,14Z]]- 6-[(2-amino-2-carboxyethyl)thio]-5-hydroxy-7,9,11,14-eicosatetraenoate | HMDB | | [5S-[5R*,6S*(s*),7E,9E,11Z,14Z]]- 6-[(2-amino-2-carboxyethyl)thio]-5-hydroxy-7,9,11,14-eicosatetraenoic acid | HMDB |
|
|---|
| Chemical Formula | C23H37NO5S |
|---|
| Average Molecular Mass | 439.609 g/mol |
|---|
| Monoisotopic Mass | 439.239 g/mol |
|---|
| CAS Registry Number | 75715-89-8 |
|---|
| IUPAC Name | (5S,6R,7E,9E,11Z,14Z)-6-[(2-amino-2-carboxyethyl)sulfanyl]-5-hydroxyicosa-7,9,11,14-tetraenoic acid |
|---|
| Traditional Name | leukotriene E4 |
|---|
| SMILES | CCCCC\C=C/C\C=C/C=C/C=C/[C@@H](SCC(N)C(O)=O)[C@@H](O)CCCC(O)=O |
|---|
| InChI Identifier | InChI=1S/C23H37NO5S/c1-2-3-4-5-6-7-8-9-10-11-12-13-16-21(30-18-19(24)23(28)29)20(25)15-14-17-22(26)27/h6-7,9-13,16,19-21,25H,2-5,8,14-15,17-18,24H2,1H3,(H,26,27)(H,28,29)/b7-6-,10-9-,12-11+,16-13+/t19?,20-,21+/m0/s1 |
|---|
| InChI Key | OTZRAYGBFWZKMX-MPWKMEBCSA-N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as leukotrienes. These are eicosanoids containing a hydroxyl group attached to the aliphatic chain of an arachidonic acid. Leukotrienes have four double bonds, three (and only three) of which are conjugated. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Eicosanoids |
|---|
| Direct Parent | Leukotrienes |
|---|
| Alternative Parents | |
|---|
| Substituents | - Leukotriene
- Hydroxyeicosatetraenoic acid
- Long-chain fatty acid
- Cysteine or derivatives
- Alpha-amino acid
- Alpha-amino acid or derivatives
- Hydroxy fatty acid
- Thia fatty acid
- Dicarboxylic acid or derivatives
- Fatty acid
- Unsaturated fatty acid
- Amino acid or derivatives
- Amino acid
- Secondary alcohol
- Carboxylic acid derivative
- Carboxylic acid
- Dialkylthioether
- Sulfenyl compound
- Thioether
- Amine
- Organic oxygen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Alcohol
- Carbonyl group
- Organopnictogen compound
- Primary aliphatic amine
- Organonitrogen compound
- Organooxygen compound
- Organosulfur compound
- Primary amine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Biological Properties |
|---|
| Status | Detected and Not Quantified |
|---|
| Origin | Not Available |
|---|
| Cellular Locations | Not Available |
|---|
| Biofluid Locations | Not Available |
|---|
| Tissue Locations | Not Available |
|---|
| Pathways | Not Available |
|---|
| Applications | Not Available |
|---|
| Biological Roles | Not Available |
|---|
| Chemical Roles | Not Available |
|---|
| Physical Properties |
|---|
| State | Not Available |
|---|
| Appearance | Not Available |
|---|
| Experimental Properties | | Property | Value |
|---|
| Melting Point | Not Available | | Boiling Point | Not Available | | Solubility | Not Available |
|
|---|
| Predicted Properties | |
|---|
| Spectra |
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00r6-7109100000-370f509d8e5778797898 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0006-9302216000-39f667b7ab5bd4020938 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0096-2008900000-b2a7ad739f2ebc335854 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-3739100000-a067c58d5e580ca6cdbf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00bc-8139000000-82a37887ecb91779e434 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-2309800000-b8590815fe190af6a61e | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fl9-2329000000-5cef54758a90e599fbdc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-9310000000-8227f0ebad6c5a52c4f8 | Spectrum |
|
|---|
| Toxicity Profile |
|---|
| Route of Exposure | Not Available |
|---|
| Mechanism of Toxicity | Not Available |
|---|
| Metabolism | Not Available |
|---|
| Toxicity Values | Not Available |
|---|
| Lethal Dose | Not Available |
|---|
| Carcinogenicity (IARC Classification) | Not Available |
|---|
| Uses/Sources | Not Available |
|---|
| Minimum Risk Level | Not Available |
|---|
| Health Effects | Not Available |
|---|
| Symptoms | Not Available |
|---|
| Treatment | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| External Links |
|---|
| DrugBank ID | Not Available |
|---|
| HMDB ID | Not Available |
|---|
| FooDB ID | Not Available |
|---|
| Phenol Explorer ID | Not Available |
|---|
| KNApSAcK ID | Not Available |
|---|
| BiGG ID | Not Available |
|---|
| BioCyc ID | Not Available |
|---|
| METLIN ID | Not Available |
|---|
| PDB ID | Not Available |
|---|
| Wikipedia Link | Leukotriene E4 |
|---|
| Chemspider ID | Not Available |
|---|
| ChEBI ID | Not Available |
|---|
| PubChem Compound ID | Not Available |
|---|
| Kegg Compound ID | Not Available |
|---|
| YMDB ID | Not Available |
|---|
| ECMDB ID | Not Available |
|---|
| References |
|---|
| Synthesis Reference | Not Available |
|---|
| MSDS | Not Available |
|---|
| General References | Not Available |
|---|