| Synonyms | | Value | Source |
|---|
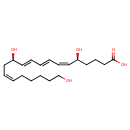
| (6Z,8E,10E,14Z)-(5S,12R)-5,12,20-Trihydroxyeicosa-6,8,10,14-tetraenoate | ChEBI | | (6Z,8E,10E,14Z)-(5S,12R)-5,12,20-Trihydroxyicosa-6,8,10,14-tetraenoate | ChEBI | | 20-Hydroxy-LTB4 | ChEBI | | 20-OH-Leukotriene b4 | ChEBI | | 20-OH-LTB4 | ChEBI | | (6Z,8E,10E,14Z)-(5S,12R)-5,12,20-Trihydroxyeicosa-6,8,10,14-tetraenoic acid | Generator | | (6Z,8E,10E,14Z)-(5S,12R)-5,12,20-Trihydroxyicosa-6,8,10,14-tetraenoic acid | Generator | | (5S,12R)-5,12,20-Trihydroxy-(6Z,8E,10E,14Z)-eicosatetraenoate | HMDB | | (5S,12R)-5,12,20-Trihydroxy-(6Z,8E,10E,14Z)-eicosatetraenoic acid | HMDB | | (5S,6Z,8E,10E,12R,14Z)-5,12,20-Trihydroxyicosa-6,8,10,14-tetraenoate | HMDB | | (5S,6Z,8E,10E,12R,14Z)-5,12,20-Trihydroxyicosa-6,8,10,14-tetraenoic acid | HMDB | | 20-Hydroxy LTB4 | HMDB | | 20-Hydroxyleukotriene b4 | HMDB | | 20-OH-5S,12S-Dihydroxy-6,10-trans-8,14-cis-eicosatetraenoate | HMDB | | 20-OH-5S,12S-Dihydroxy-6,10-trans-8,14-cis-eicosatetraenoic acid | HMDB | | 5,12,20-THETE | HMDB | | 5,12,20-TriHETE | HMDB | | 5S,12R,20-Trihydroxy-6Z,8E,10E,14Z-eicosatetraenoate | HMDB | | 5S,12R,20-Trihydroxy-6Z,8E,10E,14Z-eicosatetraenoic acid | HMDB | | Omega-hydroxy-LTB4 | HMDB | | W-Hydroxy-LTB4 | HMDB | | [S-[R*,s*-(e,Z,e,Z)]]-5,12,20-trihydroxy-6,8,10,14-eicosatetraenoate | HMDB | | [S-[R*,s*-(e,Z,e,Z)]]-5,12,20-trihydroxy-6,8,10,14-eicosatetraenoic acid | HMDB | | 20-Hydroxy-leukotriene b | HMDB | | 5,12,20-Trihydroxy-6,8,10,14-eicosatetraenoic acid | HMDB | | 5,12,20-Trihydroxy-6,8,10,14-eicosatetraenoic acid, (S-(r*,s*-(e,Z,e,Z)))-isomer | HMDB |
|
|---|
| Spectra | | Spectrum Type | Description | Splash Key | View |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-00lj-3798000000-5bb085b2304969403378 | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (4 TMS) - 70eV, Positive | splash10-004i-7400967000-a9f5c76728347ea2738b | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QIT , negative | splash10-0udi-0009000000-2b98c651c0cd0833ccef | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QIT , negative | splash10-0udi-0009000000-a681c9193735a4386b49 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QIT , negative | splash10-0udi-0009000000-8db36dce15edb0d7b2f3 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QIT , negative | splash10-0udi-0109000000-e01ef44cb36e9df489f9 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QIT , negative | splash10-0uea-0709000000-d7a7994b98cf3bfbe072 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QIT , negative | splash10-0f8b-0924000000-bb49aa4e02bfea5085c5 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QIT , negative | splash10-0il1-0910000000-8f7913074625390c6208 | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QIT , negative | splash10-0di9-0900000000-a87a75ca80ddc1745a1f | Spectrum | | LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QIT , negative | splash10-0a4i-1900000000-972b13fd5c8b54c14aa2 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00kr-0019000000-fed023a2c990a5c6753f | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014r-1269000000-22fedd5f102230a634a7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00tp-6491000000-bfc9e7fd9147e861f375 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0009000000-e84044e33fbc237fabfc | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0uei-1129000000-f307e44b0f4e538946e7 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9341000000-49f0ced38039afa26a80 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014r-0019000000-f4220ba5e589bf99a9ea | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-014i-1139000000-9334bf38981381cc7a00 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-002g-9532000000-8d635fd79d5a07bfbaa8 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0009000000-9b61e666ea58e89197b4 | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udi-1229000000-f6625299fa9cf1f41bbf | Spectrum | | Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a6u-6298000000-f0b013babab01e4ad7b8 | Spectrum |
|
|---|